The paradigm of biological psychiatry—in short, the view that mental disorders are diseases of the brain—is essentially based on three assumptions: First, that the brain is the organ of the mind; second, that psychoactive substances or electrical stimulation of the nervous system alleviate the symptoms of mental disorders; and third, that parents genetically pass on a predisposition to mental disorders to their children.
It is now common knowledge that there is no reliable biological test, or biomarker, for any of the hundreds of disorders in the DSM-5. I assume that our mind is embodied, yes. That is why psychoactive substances affect our feelings, thoughts and behavior. Many people know this from their own experience, even if it is only with caffeine, alcohol or tobacco. However, mental disorders are not concrete things that can be found with a brain scanner or treated with medication like a bacterial infection with antibiotics.
Much has already been written about these points, for example in my book on mental health and substance use (open access). I will therefore focus here on the third pillar of biological psychiatry: genetics.
Our bodies are also biological and the result of a long evolutionary history. Genes are permanently active and regulate the production of proteins from which larger structures are built. In this sense, everything is somehow genetic. And genes also play a role in our behavior. Eric Turkheimer, professor at the University of Virginia, formulated the first law of behavioral genetics 25 years ago: All human behavioral traits are heritable.
Nevertheless, the heritability estimates that are still frequently reported exaggerate the importance of biology for psychiatry. In this essay, I explain why this is the case and why it is not possible to clearly separate the role of genes, environment and psychology.
How to measure the genetic contribution
We have probably all heard statements such as “intelligence, schizophrenia or ADHD are 80 percent heritable” more than once. Scientists also regularly quote such figures. But only few understand that these statements do not prove a strong genetic influence. Leading human geneticists and evolutionary biologists have been explaining this for decades.
However, this message is hardly taken up, probably because it does not fit in with the biological-neuroscientific zeitgeist. In order to explain the criticism, I will not go deep into the mathematical basics here. Instead, I will briefly describe the most common measurement methods and then use concrete examples to expose the misunderstandings about heritability.
Two methods are particularly important for genetics in psychiatry: genome-wide association studies (GWAS) and twin studies. For both, a distinction is made between the “genotype” and the “phenotype.” The latter refers to the appearance of a living being and can be a physical measurement such as height, but also a psychological characteristic such as intelligence or the severity of a depressive disorder.
In the 1990s, genetic studies were still very complicated and expensive. At that time, scientists had to select only a few so-called candidate genes, based on what they thus far knew about genetics, and to investigate their variation between people. Would certain genotypes occur more frequently together with a phenotype? For example, are people diagnosed with depression more likely to have gene variant XYZ than ZYX?
Genome-wide association studies
Because it was already assumed at that time that mental disorders were highly heritable, the small and inconsistent results found in these studies were disappointing. For this reason, genome-wide association studies, known as GWAS, were called for in ever larger samples. These allowed scientists to not just investigate a selected few, but all genes at the same time. There are now studies in which the data of tens, hundreds of thousands or even millions of people have been examined.
Two findings are clear, as shown in the following table: The larger the studies become, the more genes stand out as statistically significant; however, this is due to the mathematical fact that smaller and smaller differences are found with ever larger samples. Therefore, the explained variance remains rather small even in these newer studies. This means that even if 270 risk genes for schizophrenia have now been found, they can only explain a small proportion of the symptoms.
| Malfunction | Number of persons (approx.) | Number of genes | Explained variance (%) |
| Anxiety disorders | 200,000 | 6 | 0.5 |
| ADHD | 55,000 | 12 | 5.5 |
| Autism Spectrum | 53,000 | 5 | 1.4 |
| Bipolar disorder | 413,000 | 64 | 4.6 |
| Anorexia | 73,000 | 8 | 1.7 |
| Depressive disorder | 1,154,000 | 178 | 1.5-3.2 |
| PTSD | 207,000 | 3 | 0.15 |
| Schizophrenia | 306,000 | 270 | 7.7 |
| Tourette syndrome | 14,000 | 1 | 0.8 |
Table: According to Giangrande and colleagues. Although large studies have now been completed for many disorders, only a few percent of the differences in symptoms are usually explained genetically (explained variance). The number of genes represents the number of locations on the genome where statistically significant differences were found.
It is often forgotten that the “A” in “GWAS” stands for “association,” for a common occurrence. However, every introductory course in statistics teaches you that this does not prove a cause-and-effect relationship. For example, in some rural regions there are more storks and the birth rate is higher. However, this is because both storks and people with many children prefer a rural environment to cities—and does not prove that storks bring the babies.
This is also shown by the fact that the contribution of genes found in such studies decreases when the researchers include more psychosocial factors. In a corresponding study led by Jim van Os, a long-standing schizophrenia expert at the University of Utrecht in the Netherlands, differences in genes only explained 3 percent of the symptoms. In contrast, social circumstances and the environment played a much greater role, accounting for 30 and 24 percent respectively.
Interim conclusion: The GWAS that are so often praised today do not confirm any major influence of genes, in particular no cause-and-effect relationship, and are dependent on the inclusion of other factors.
Twin studies: Pragmatism and mistakes
Twin studies are cited as further evidence of the importance of genes for psychiatry. After all, we know that monozygotic (MZ) twins are almost 100 percent identical, whereas fraternal or dizygotic (DZ) twins are only about 50 percent identical. By comparing how often a phenotype such as schizophrenia occurs in monozygotic and dizygotic twins, it is possible to estimate heritability as opposed to environmental influences. This leads to the aforementioned claims such as “80 percent heritable.”
However, it is rarely explained that twin studies use statistical models whose assumptions are controversial. For example, it is not taken into account that identical twins are treated differently by their environment than fraternal twins due to their strong similarity. In addition, errors occur during data collection. The clinical psychologist Jay Joseph has published several books and articles here on Mad in America about the limitations of twin studies.
Many researchers pragmatically ignore such limitations and errors. The twin studies deliver the results that fit the zeitgeist: The aforementioned problems lead to an overestimation of genetic and underestimation of environmental influences. Incidentally, the large differences between the results of the GWAS and the twin studies are now explained by the fact that there must be a “hidden heritability.”
Instead of repeating the criticism that Joseph and other researchers have already formulated in more detail, I would like to discuss a few specific examples here. The so-called concordance rates are easier to understand than the heritability estimates, even without in-depth mathematical knowledge. These describe how often the second twin has a diagnosis such as schizophrenia if the first twin already has it.
Get real: concordance rates
A large Danish study investigated the heritability of schizophrenia. Data from around 32,000 pairs of twins born between 1951 and 2000 were analyzed. From this, the researchers calculated a heritability of 80 percent and interpreted this as a sign of “a substantial genetic risk” of this psychological-psychiatric disorder.
Let’s imagine for a thought experiment that you meet one of two identical twins and find out that he has been diagnosed with schizophrenia. You have just read that the heritability of this is 80 percent. Later you also meet the other twin. In your opinion, what is the probability that this twin will have the same diagnosis? (What is the concordance rate?)
Laypeople (but also many scientists) would probably now think that the second twin was also diagnosed with schizophrenia in 80 percent of cases. However, that is incorrect: according to the study, the concordance rate for schizophrenia is only 33 percent for MZ twins and only 7 percent for DZ twins. Mind you, the former are genetically more or less identical, while the latter are still around 50 percent identical! This should be taken into account when people with psychiatric diagnoses are advised about their family planning in genetic consultations.
Causes: Biological or psychological?
What has been shown here using the example of schizophrenia applies in a similar way to all mental disorders. It should be borne in mind that schizophrenia is already considered one of the most biological disorders, as can be seen in the following illustration.
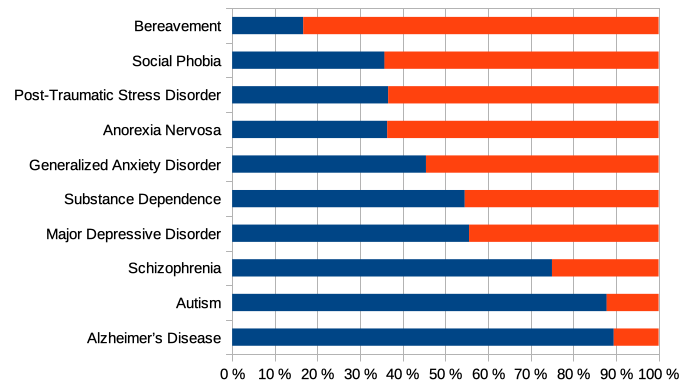
Illustration: Biology or psychology, which is considered the cause of mental disorders? According to the study by Ahn and colleagues, experts considered the biological component to be particularly high in schizophrenia, autism and Alzheimer’s disease (blue), while they assumed a particularly high psychological component in bereavement, social phobia and post-traumatic stress (red). Image from Schleim (2023), CC BY 4.0.
As a final objection to heritability estimates, I will explain that they themselves depend on the environment.
Understanding heritability
While the linguistic meaning of “heritability” clearly suggests a genetic contribution, this is unfortunately not necessarily the case. To illustrate this, we must understand that researchers try to explain differences in the phenotype due to differences in the genotype.
For example, are taller people or people with more severe depressive symptoms more likely to have genotype XYZ than ZYX? If so, then these differences in phenotype are said to be explained by differences in genotype. Results from large GWAS can be seen in the table above, in the column for the explained variance.
The misunderstanding of heritability estimates is often explained using examples such as the following: Let’s assume a farmer has a wheat field. She sows the wheat, fertilizes the soil and irrigates it. However, due to a period of drought, the farmer’s water supply runs out after a while.
She decides to use the remaining water only for the left half of the field. This will then produce the full yield. The farmer expects a better harvest than if she irrigates both halves insufficiently. The wheat on the right half will then wither, as shown in the following illustration.

The important thing now is that we have the same environment on the left and right halves, apart from the irrigation. Within the left-hand field, there will be differences in the growth of the plants, which must then be (almost) exclusively genetic—the environment is identical. This means that the existing differences are explained genetically, the heritability is high.
The wheat on the right is stunted, but here too there are small differences in growth. These are also (almost) only due to genetic differences. So heritability is also high here. But if we compare the left and right fields, we see huge differences. Despite the high heritability, this is due to irrigation, an environmental characteristic.
In general, it can be said that the more uniform the environment, the greater the estimated heritability. This is because the differences that then still exist must be genetically determined. You can also imagine this in humans: If, for example, everyone is equally poorly or equally well nourished, the differences in body size will be mainly due to genes and the calculated heritability will be high. However, if there are greater differences in nutrition, part of the differences in size will be explained by the environment and part by genetics. The heritability is then lower.
In a study led by the aforementioned psychologist and geneticist Turkheimer, such an effect was demonstrated in reality: For children from poorer backgrounds, differences in IQ were explained primarily by environmental effects, whereas for the wealthy they were more strongly explained by genes. This could mean that the latter already lived in an environment that was ideal for intelligence and that the differences that then still existed were due more to genetic differences—as with the wheat on the left-hand side of the field in the example.
Interim conclusion: Heritability estimates exaggerate the genetic influence due to incorrect assumptions and errors in data collection. The true concordance rates suggest a much smaller genetic contribution, in line with the newer GWAS results. In particular, heritability estimates cannot be applied to individuals and are only meaningful for a specific population in a specific environment. They do not allow us to infer the relationship between genotype and phenotype in another population in a different environment.
Old knowledge
What I have just explained is old knowledge of genetics. The frequent misinterpretation of heritability estimates in psychiatry and the media is based on a linguistic misunderstanding. A few years ago, two researchers even described heritability as “one of the most misleading [terms] in the history of science” in an essay worth reading and warned that “continued use of the term does enormous damage to the public understanding of how human beings develop their individual traits and identities.” The misunderstanding plays into the hands of those who want to research mental disorders genetically.
As early as the 1970s, Stanford biologist Marcus Feldman and Richard Lewontin from Harvard University criticized the concept of heritability in human genetics in a much-cited publication in Science. They concluded that this approach is like trying to explain the mechanism of a clock by observing the hands and their ticking.
In the 1990s, philosophy and psychology professor Ned Block of New York University discussed the widespread misconceptions about heritability and the danger of racist conclusions. The reaction to the then much-discussed book The Bell Curve, which claimed that the heritability of IQ in white people was 60 percent and that black people had poorer genes, was also discussed in the media at the time.
Perhaps such thoughts prompted dozens of renowned ADHD researchers not to quantify the genetic contribution in a widely cited consensus paper. In particular, they do not report a heritability estimate. And rightly so! On the website of his ADHD Evidence Project, Stephen Faraone, one of the authors of the consensus paper and professor at SUNY Upstate Medical University, unfortunately informs the public differently: “Genetics contributes substantially to ADHD, with a heritability of approximately 80%.” We now know that this statement is misleading.
But if it is not possible to calculate the genetic influence in this way, how can it be done? It is generally recognized that many characteristics are based on a gene-environment interaction, including in psychology and psychiatry. Here, however, we are dealing with circular causality: gene, person and environment interact endlessly.
Genes influence how we feel, think and behave, yes; they partly explain why one person has a greater tendency to be impulsive or depressed than another. However, the environment and our experiences in it have a continuous effect on our body—and this in turn regulates gene activity, as is currently being researched in epigenetics. We have to say goodbye to the idea that we are dealing with mathematics in the form 2 + 2 = 4.
In order to obtain reliable results on the genetic influence, we would need experimental interventions: Individual genes or gene segments would have to be switched on and off or reprogrammed. The consequences for people’s health would be dramatic. In addition, the test subjects would have to be kept in controlled environments, like in a zoo. This is all forbidden for ethical reasons. It is done in animal experiments, but their results only apply to humans to a limited extent. In fact, heritability estimates only make sense in animal and plant breeding.
And even genes strongly determine a particular trait, they are not necessarily our fate. Think of rare monogenic diseases such as Huntington’s or spinocerebellar ataxia: If you have the wrong genotype, you are (almost) 100 percent likely to become ill. At the same time, however, this also defines a therapeutic goal.
This means that even in such a case of strongest genetic determination, social factors such as the availability of medical help can be decisive. This brings me to the last point, the social consequences of misunderstood research.
Social consequences
Until the 20th century, the concept of heritability was misused to justify the prevailing social conditions: Those in the upper classes simply had the better genes. Social Darwinism, inequality and colonial oppression were thus presented as a natural order. Even today, researchers criticize “biological essentialism,” which can lead to the justification of exclusion and racism.
This is how misunderstood biology becomes politics: For example, if intelligence were strongly genetically determined, couldn’t education spending be cut? But, as we have seen, even then the remaining percentages could still make a big difference. You could just as well argue the opposite and provide more social support for the naturally disadvantaged in the interests of equal opportunities.
Regarding mental disorders, pharmaceutical treatments appear better if genetic causes are assumed. However, according to current knowledge—apart from rare individual exceptions—this only involves slightly increased risks. Nevertheless, the discourse on diseased brains and genes threatens to obscure the significance of social and environmental influences such as poverty, serious life events, migration, stress and trauma.
The paradigm of biological psychiatry mentioned at the beginning, with its image of disorders as brain diseases, is so persistent because the three basic assumptions each have a true core: Mental processes are embodied and can therefore be influenced pharmacologically or electrically. The risk of a mental disorder is genetic and heritable in a weak but not exactly quantifiable sense. As I once put it myself: Mental disorders are brain disorders—and they are not.
We now know that the many billions of research dollars that have been invested in biological psychiatry since the 1980s have achieved little: There are no reliable biological tests, new revolutionary therapies have failed to materialize and the vaccinations against mental disorders once promised by the former director of the National Institute of Mental Health, Thomas Insel, for the year 2020 are not even on the horizon. At the same time, many countries are facing an unprecedented mental health crisis.
My point of view is that the disorders are only biological in a weak sense. Prevention and therapy should focus primarily on the psychosocial sphere. This is not only a scientific, but essentially a socio-political task. Stopping the spread of misleading heritability estimates would be a step in this direction.

This “problem” exists because we are immortal spiritual beings who have built our minds as they currently exist over thousands of lifetimes, some of those lifetimes probably in very different bodies than we use now.
Of course environment plays a huge role in how we react in this current body, but our minds have been with us through countless different environments in many different places. These are the findings that academic psychology refuses to even grapple with. They pretend these findings don’t exist, but indeed they do.
Report comment
Well, said I totally agree
Report comment
I don’t know how you live and find meaning in the human world. It’s just like being a bird in a cage or a cat confined in a little apartment just with one miserable human being for company. It is worse then purgatory. You have no leisure to be or to understand yourself because you live in social world of word, idea and effort, in constant striving with it’s endless frustrations, in constant craving ever endlessly seeking but never finding fulfilment, as fear governs the contours of your life making such fulfilment impossible and you call this life. Because in the endless deferral of fulfilment there is this mirage we call ‘hope’, without which you could not live – but this hope is never realized, and if you saw this fact, you could no longer live your stupid life. And you don’t know what life is really about. Who does? Only the free and natural creature understands what life is about. You know what you are striving for – recognition, social status, to be appreciated by others, but for what exactly? Some vague fulfilment. But that’s because you don’t know what life is about. And if you ever attained all your confused and limited goals and dreams in life you would see them for what they are – insufficient. There is no social activity or language that can articulate your longing so you don’t know what this longing is for and will never find out in the human world. So what is to be done?
You don’t have to live in the human world. Just see it for exactly what it is and then already you are out of it, for the same reason that if you found your head in a crocodile’s mouth you would withdraw it before it snapped shut. And you would then say to others – I don’t know how you can live in the human world. I don’t live in the human world – I live in the clarity of consciousness, of perception, and I watch the human world from that consciousness, and this is what I see. Nothing but a wasteland of socially conditioned, confused, aimless human beings living in vein hope which drives them through a meaningless existence sculpted and defined and circumscribed by the collective coercion of power and social history, producing unfulfilled human beings who like factory farm animals fuel the meaningless existence we are all trapped in. Being unfulfilled and constrained you will strive and strive to find fulfillment in the vampiric structures created in order to exploit and extract from you, and this you call life. You have become what you have become – utterly dependent on vampires, on all those with power, dependent on a socially constructed and extractive process that destroys you, and you have become all this because society has conditioned you to become this so those with power who run society can easily entrain you into their systems of extraction, and then consume your life, quickly or slowly, for every kind of profit – social, psychological, financial and spiritual. If you are successful in social life they will instrumentalize your life for profit. And if you are poor or powerless they will plunder you and crush you with demands. The only thing anyone can do in this situation is to see this obvious fact, so I invite you simply to see it. Because the seeing of this fact IS the understanding of it, and that understanding will act. Otherwise you stand more to gain from suicide then you do from this life, just like any other factory farm animal, for example me and you.
It’s a factory farm society producing production line human beings, of every kind – spiritual, socialist, Buddhist, anarchist, environmentalist – it doesn’t matter. They are all socially mass produced identities and activities. None of this is apart from the total system which is destroying us all and the Earth. There is no social or intellectual way. Seeing things as they are is neither social nor intellectual – it makes the latter irrelevant. And it is the only solid ground left on Earth. And I vomit this out to people today and every day, because it makes me so sick. I feel like I will drown the Earth in sick. And all the internet forums including MIA for me are just so many sick bags.
Report comment
But heritability cannot explain the most devastating and universal and factual mental disorder that never made entry into the DSM because firstly it is the only valid mental disease to note and secondly all psychiatrists and mental health professionals and psychopharmacologists and politicians and business people and doctors and academics have it, as do almost all citizens. It is the enslaved, socially conditioned mind that has destroyed us all and hollowed out our lives, making it functional and without deep meaning. And it traps you first by instilling in you an existential insufficiency which makes you strive, strive, strive for your desires, which is like whipping a horse to make it gallop through the the socially designed and designated corridors of prescribed socially conditioned activities. That is how they trap you, so we have to understand this trap and when we do we evolve out of it. Please let me explain.
Some will know this and some will not: there is an evolution of human consciousness, but IT IS NOT A COLLECTIVE PROCESS, in the sense that most people are obviously not going through it now and most never will. Most people are not evolving but degenerating, falling further and further into conflict and confusion, less and less able to make sense of their circumstances and their lives let alone their world, which IS their life in macrocosm – they are not separate. And the reason most people and most things are degenerating and falling apart is because most people pursue what they want, rather then first pursuing an understanding of what actually is, and when they are hopeful about getting what they want they can’t see the dysfunction in it.
But if you use your brain to pursue what you want without first understanding what is, obviously you create confusion, conflict, and destruction because of that ignorance as your self-striving efforts run into contradiction to what actually is, which you end up blaming. It is like a young fawn eating all the plants it wants to without first understanding which is poison, only our social world is far more unnatural, full of dangers and ignorance, hence almost EVERYTHING IN IT is poison. And if you don’t realize that it is you who in your ignorance are poisoning yourselves with the toxins of social life, you will blame others for all your ills. You will say it is because my boss stood in the way of my pay rise, or my parents didn’t love me enough which is why I suffer, or it’s because of immigrants or the Arabs or the Jews or the Hindus or the Muslims or the Prime Minister – but the true cause of your suffering is your innocence about life, your ignorance, which was maintained because instead of using your brain to understand life you used it merely to try and grab what you want.
So in your confusion you blame, blame, blame, which is based on false socially conditioned judgement, and a blame or judgement can never be the fact. The fact is the fact. The judgement of the fact can never the the fact, nor the blame it gives rise to. So blame is proof that you haven’t understood what is, because in understanding there is no blame, just seeing things as they are enabling an intelligent response to it. And when you blame you alienate reality, you alienate others, you alienate yourself, you push away an understanding of life in exchange for the temporary emotional satisfaction of making it someone else’s or something else’s fault which convinces you that you don’t need to understand things any further. Hence you seal in your confusion in a tomb of judgement making it’s healing and resolution impossible.
I am not saying there are not destroyers, I am not saying people and society is not violent. I am simply saying that you only become a victim of these things through YOUR OWN IGNORANCE OF LIFE, because if we understood life we would have acted intelligently in response to them and not been a mere helpless, impotent victim of them. And almost everyone in this society is such an impotent, helpless victim struggling in confusion and recrimination, unable to see things as they are. And that is why they are degenerating, disintegrating, and losing themselves all the more surely. Now we simply do not see the gravity, the fundamental importance of all this in yours and everybody else’s life, including all of our children who only have dangerously ignorant and confused parents to guide them. To be lost, to be damned, is to become so adrift from the truth of things that you no longer have any clear sense of what or who you actually are. And then you are at the mercy of all the forces of power surrounding you. But to be clear and to be yourself and to be free is being what you actually are and seeing and understanding yourself in that freedom. In seeing and understanding things as they are there is no blame or judgement, because there is no need to judge or blame – you understand what took place and can respond intelligently to it. That understanding of life therefore brings freedom rom self-judgement and judgement of others, and clears away any sense of blame. You don’t need to blame someone who hurts or is violent to you but merely uncover and see that violence for what it is and walk away. That is not blame but intelligent action.
So this journey of understanding things as they are IS liberation from the false, from the violent, from the untrue, from the domination and all the traps of seeking pleasure, desire and avoiding your fears rather then being brave enough to open your eyes and see things as they are. All human beings, children and adults, who don’t understand this in their life times will die in confusion and ignorance, and there is no reincarnation for a humanity that has reached the end of it’s road and must now leap into a new level of conscious being. You will be like dead leaves shed form the tree of life and nobody will be able to save you because salvation comes about only through the true understanding of life which nobody else can give you. You have to start seeing things as they are and stop judging. Otherwise ultimately you are doomed. There is barely anything else worse saying to most people today.
Report comment
“Eric Turkheimer, professor at the University of Virginia, formulated the first law of behavioral genetics 25 years ago: All human behavioral traits are heritable.”
The ref given for this is closed access so I could not confirm the dubious accuracy of this quote. As it reads, it implies traits are 100% heritable. As I recall, he was pointing out, correctly, that all traits are moderately heritable.
“The reaction to the then much-discussed book The Bell Curve, which claimed that the heritability of IQ in white people was 60 percent and that black people had poorer genes, was also discussed in the media at the time.”
Please supply the exact quote from TBC that states this. The book was about societal effects of low IQ.
Report comment
The quote about heritability is literally how Turkheimer formulated the first law of behavior genetics, e.g. in the paper referenced here. I myself prefer to publish as much open access as possible – and even don’t have access to all scientific papers I need via our university’s library.
When I looked up the paper on Google Scholar right now (just pasting the title into the search field), there appeared a link (“[PDF] sagepub.com”) on the result page that allowed me to download the article that might also work on your computer.
But regarding your interpretation of the first law: I think this shows once more how fuzzy and dangerous the heritability concept is, how easy it can lead to misunderstanding.
I’d formulate it thus: As we are also physical, biological beings with an evolutionary history, everything has a genetic basis – genes being a kind of “blue print” which controls the production of molecules that literally constitute our organs, tissue, bodies. But as gene activity is itself subject to the environment and there is causality on the higher level (e.g. organs), genes can only explain part of the variability of the organism’s phenotype and behavior.
The information about The Bell Curve can also be found in the article by Ned Block that can be accessed via the link.
Report comment
In any controversial topic, it is essential to quote from primary sources. We only have to look at social media to see what happens when opinions are repeated without checks, and alternative facts proliferate. So, can we all have here the brief original quotations?
Report comment
The law of behavioral genetics is already – I say it again – a direct quote from the linked article.
The statement about The Bell Curve can be checked in the linked source, too, a book review by a renowned professor in 1995. The argument is developed throughout the book and not in one single sentence, as some here seem to assume.
The genetic racism in the book led to a huge discussion back then, which is well documented in various online and offline sources. However, that’s totally not the point in my essay, but that misunderstandings of “heritability” were already discussed a long time ago, e.g., by that professor in the 1990s (follow the link).
Put differently, the controversy here is not whether IQs differ between blacks and whites, but whether the “heritability” concept is misleading.
P.S. If you’re interested in the discussion of genetic racism in the 1990s, you can, besides the references I quoted, best read Chapter 13 in The Bell Curve, especially the section Genetics, IQ, and Race, starting on p. 295 – and judge for yourself.
Report comment
To No One:
I get the feeling that you don’t suffer from any mental health issues so therefore you are not currently a victim of the US brainwashed mental health system. I’ve been begging for help from psychiatrists, psychologists and different medical doctors for over 20 years as I have been overmedicated, with mental and physical symptoms getting worse. I’ve received multiple mental health diagnosis and can not get a straight answer from anyone. The human body is very intricate comprised of multiple complex systems and needs to examined as a whole and not broken down into parts. Everything needs to be factored in. The USA has been influenced by our government including the FDA, CDC, big pharma, and so many other agencies that I don’t need to list. It has influenced the way our doctors are trained and what treatment are to be used, it has influenced farming, what is grown today is not as nutritious. So many of the additives in our foods, beauty products and more are illegal in many other countries. I can go on. My main point is if the article and the following comment has nothing to do with you, specifically if you don’t suffer from mental health issues and are being treated with these medications or treatments that are overused and sometimes cause more harm than good please express your feelings elsewhere. This is not the place to judge. It’s a place to gather information which can help you make a more informed decision about your treatment options. If you have a question, wonderful, or even just express a general opinion. It’s not a forum to attack someone for their thoughts or feelings.
Report comment
I spent wasted years on antipsychotics and antidepressants because like you I believed the propaganda. Now I’m passed diagnoses and pills and into a freedom which is yours for the taking. It involves you being you. Can you handle it?
Report comment
Thanks for this article.
The psycho/medical fraternity have an intellectual and vested interest in the atomisation of our mental-health difficulties.
There are, fortunately, others who have provided evidence to substantiate the common-sense idea that more egalitarian societies improve general well-being.The published works of Richard Wilkinson and Kate Pickett are examples of this.
Report comment
I haven’t read the whole article but want to add that heritability estimates make a basic mathematical mistake. They assume that genes and environment have completely separate and additive effects. Empirically this is obviously false, because early environments can interact with the genome in ways that constrain the ways the same genome can be leveraged later on in different environments. But even more than this it is false because in the absence of genes the environment has ZERO effect, and in the absence of the environment genes have ZERO effect. That makes the separate effects of both genes and environment in isolation from one another 0% each, whereas together genes and environment account for 100% of the variation (forgetting for the moment that this dualism isn’t well made to begin with). It’s true that if the monozygotic concordance rates were 100% or close to it, there would have to be a very aberrant environment for an outcome not to occur, but still an environment. And they are not anywhere near 100%, which means in EVERY INDIVIDUAL CASE it is the complex interaction between environment and genetics (though again I don’t think this is necessarily a good dualism) over time that produces the outcome. It doesn’t really matter at the individual level how much one variable accounts for the variance in the population, looking at any one person it will still be interactions that make the difference.
Report comment
I, for one, would not like to pick an argument with the very clever statisticians who have come up with heritability estimates, so any problems are with their interpretation. And, who says G and E must add up to 100? Could there not be a large random component?
Are you able to help out with providing primary source quotations as per my comments on May 19 and 20?
Report comment
I’ve added here another comment regarding these questions and suggest, again, that you do your own research if that’s a topic you find interesting: https://www.madinamerica.com/2025/05/heritability-explains-less-about-mental-disorders-than-you-think/#comment-317742
Report comment
Don’t see this as a reply to my comment, really. That you don’t want to pick an argument with clever statisticians doesn’t mean they’re not wrong. Here’s a quote by Dennis Noble, if you need the evidence to come from someone who is on the record as being clever:
“Complex biological functions have correspondingly complex causal logic. Be wary therefore when someone tells you that a particular fraction of a function or disease is due to a particular cause, such as gene mutations or the environment. The fractions of causation are dependent on each other. If any cause is a necessary cause, the fractions of causation by all the others will fall to zero if that cause is absent. It is a common mistake in reductionist accounts to attribute particular percentages to genetic and environmental factors. When the processes depend on the interactions between different causes and their consequences, the outcome will not be a simple linear sum of correlations; it will be difficult to predict and can even be counter-intuitive.” – Dennis Noble, Dance to the Tune of Life
Wouldn’t bother me if there were random effects as well, though I’m not entirely sure what they’d be random with respect to. Environment is such a broad term to begin with, you could say that mold exposure from a sick building is random with respect to parenting behavior but something being ‘random with respect to the environment’ would be hard to define. But even then the effects wouldn’t be merely additive.
Report comment
Thanks, that’s a great quote and another way to formulate my thought that “it’s not as simple as 2 + 2 = 4”. I’ve downloaded the book and will have a closer look at it and the idea of “biological relativity”.
Report comment
“Be wary therefore when someone tells you that a particular fraction of a function or disease is due to a particular cause, such as gene mutations or the environment. ”
But what if you have a gene mutation that’s enriched at epigenetic sites (when combined with childhood adversity predicts depression), causes sensitivity to stress, low Folate, Vit B12, Vit D, elevated homocysteine, oxidative stress, purine and lipid abnormalities and impaired detoxification ability.
Then they get depressed and you treat them with drugs like SSRIs, antipsychotics and anticonvulsants that do the EXACT SAME THING, lower Folate, VitB12, Vit D, increase homocysteine (which causes leaky gut, endothelial dysfunction, insulin resistance),
oxidative stress and affect gut microbiota, purines and lipids.
As a matter of fact, the NIH considers MTHFR mutations as a RISK FACTOR in the use of SSRIs.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5874849/#pharmaceutics-10-00036-t001
The result would be hyperhomocystenemia, impaired methylation, inflammation, mitochondrial damage and neurotoxicity.
As Robert Whitaker said, suicide prevention strategies that increase suicide.
Report comment
Ryan,
If the genetic part (MTHFR mutations) and the environmental part (physical/sexual abuse) cause the same things: alterations in one-carbon metabolism, mitochondrial dysfunction, oxidative stress, and inflammation, then trying to figure out how much each contributed is a waste of time.
https://pubmed.ncbi.nlm.nih.gov/36987752/
Report comment
While much has been said about how heritability estimates fail to account for the complex interplay between genes and environment, what often gets overlooked is a more basic, methodological mistake: assigning causality to genetics without adequately controlling for environmental factors.
Many of the heritability studies used to support genetic causation don’t sufficiently isolate or account for early life conditions that may shape both the expression of genes and the developmental outcomes we later label as “disorders.” If two identical twins are raised in the SAME household, exposed to the same parental stress, community violence, or socioeconomic hardship, and both develop ADHD or autism, we can’t attribute this solely—or even primarily—to their shared genes. Shared environment remains a massive confounding variable.
Yet studies often draw conclusions about heritability as though those environmental influences have been cleanly filtered out. They haven’t. In reality, trauma, neglect, relational instability, and even cultural narratives all play a role not just in behavior, but in how the brain and body develop. These influences are embedded early and operate through epigenetic mechanisms, shaping gene expression over time. But heritability estimates treat those embedded effects as if they were just “noise.”
The result is a kind of scientific sleight of hand. We observe a correlation between genes and outcomes—but because we haven’t rigorously accounted for environment, we assign cause to genes. In any other field, this would be called a failure to control for confounding variables. In psychiatric genetics, it’s called evidence
Report comment
And we have to remember that in EVERY case, the prenatal environment was more or less identical for both twins.
Report comment
But in their past lives they could have been totally different people. Another “confounding variable.” And I believe most of the twin studies have not been rigorous enough to prove anything, either. Criticism of those studies goes back at least as far as 2014 on this website.
Report comment
You mean they could have had totally different experiences, I think. The idea of past lives assumes the same essential person passes from one life to another, does it not? They might assume different identities, but the person him/herself would be the same person or else reincarnation would hold no real meaning.
Report comment
I won’t get into a dissertation here, Steve. But basically what the research tells us is that decisions made many lifetimes ago can stay with us and affect our current life.
A person (spiritual being) is capable of a lot of diversity of personality, but if they are still tied to some decision they made long ago, it can really screw them up in the here and now.
Report comment
Not arguing that point, merely that they’d have to be the same person (spiritual being) in a past life in order for a past-life decision to affect them in the present. You said they might have been a “different person” in the past and I was taking issue with that. Picky point, I admit.
Report comment
Yes of course Steve.
By “different” I meant different sex, different race or language, different occupation, different name of course … things like that.
Report comment
Exactly. Same person, differing identities. We’re on the same page.
Report comment
I agree – which raises the question why such estimates, despite being biased and wrong, are made and being cited for so long in that area of research. (I guess, but cannot prove: because otherwise people with career interests in these fields would have to admit that their method won’t deliver what they promise to deliver, which is what we have been witnessing for decades.)
Report comment
Stephan you should also look into/think about how many of the folks that get labeled with psychiatric disorders have other biological issues. Let me elaborate on that so I’m not misunderstood: there is no one common biological profile for any psychiatric condition. Let alone any specific genetic abnormality. But they can be understood to be grouping together ‘symptoms’ with a vast multiplicity of possible underlying causes. Just a very simple example, but people who are sleep deprived look very irritable and spacey, they might look like they ‘have’ ADHD. How many of them have sleep apnea as a part of the causality/etiology of their irritability and spaciness? Now that’s not to say that ADHD is even one delimit-able phenomenon — probably not — or that psychosocial stressors and developmental trauma can’t have the same manifestations — they certainly can. But I think we do ourselves a disservice when we say not genetic therefore not biological. It’s yet another unfortunate effect of regarding these symptom/experience profiles as discrete biological entities with single neurobiological causes that are directly related to genetic abnormalities, that we are not looking at the other biological causes in addition to the other psychosocial causes. I can tell you for sure that my chronic vertigo caused me no shortage of anxiety and yet if I walked into a psychiatrists office they’d treat the anxiety as unrelated and distinct.
Report comment
It’s not that physiology can’t cause any of these conditions. It’s that calling the phenomenon “ADHD” assumes a unitary cause and/or treatment. I wonder how many case of sleep disturbance are dismissed as “ADHD” every year? And apparently some kids are “cured” by simply waiting a year to begin formal schooling. Can’t think of two more divergent causes. So how can we even talk about “ADHD” as if it is a THING in itself, when it’s really just a heterogeneous collection of phenomena that share only attentional difficulties as a part of the phenomena. We are clearly far better off not to use that term and to treat sleep apnea or developmental delays or even not treat ANYTHING but simply wait for the kid to grow up a bit!
Report comment
I haven’t thought about the possible relationship between SLEEP DEPRIVATION and ADHD symptoms before (also @Ryan).
Now it seems obvious to me that when people are sleep deprived, that they will be less able to pay attention, that they will tend to be less inhibited (thus more impulsive), may get bored faster, which might make paying attention even more difficult etc.
The link was more obvious to me for depression. Take a look at it’s DSM criteria (major depressive episode) and then ask yourself how many symptoms may be due to sleep deprivation (which already is one of the symptoms in itself). Lack of concentration? Lack of energy? People will move less? They cannot enjoy their daily activities any more? (Physical pain, though not an official DSM criterion of this disorder, often correlates with it.) They will gain weight? Realizing all of this, they will feel depressed? And if this persists for a long time, they may even question their life’s worth and ask themselves whether being dead might be better?
I’m not saying that all cases can be explained like this, but it seems obvious to me. Serious life events remain the major risk factor. Not in all people this triggers sleep deprivation. Some people actually sleep much more than normal.
Whether or not this kind of reasoning also applies to ADHD: Clinicians concede that there’s also a lot of “comorbidity” between ADHD and major depressive disorder (besides other conditions). Which, generally, undermines the distinctive validity of these categories, at least to some extent.
Report comment
These categories have no distinctive validity, IMHO. “Comorbidity” is a code word for “people don’t neatly fit into these arbitrary categories.” From a scientific viewpoint, these DSM “diagnoses” are very close to meaningless.
Report comment
For sure I agree with all this. I think the major point I was trying to make which it seems we agree on is that when looking at people who are really struggling in ways that the DSM (clearly very poorly) describes, we need to be thinking more holistically about multiple possible and interrelated causes, and less reductively, because there is not going to be any one isolable cause with no systemic effects elsewhere that can be attributed to any and every individual struggling in ways that look categorically similar. Your point is well taken about serious life adversity often being the cause of sleep deprivation. In fact I think the evidence is quite good that psychosocial stressors can be causally related to physical illnesses and not just the other way around. Incidentally since I stopped talking to my abusive parents my hashimoto’s antibodies have all but normalized (when I was talking to them I was fast approaching hypothyroidism). To be clear ‘stop talking to your parents’ is not going to be advice that can be generalized as therapeutic for everyone with hashimoto’s. But there is a vast body of evidence relating childhood adversity and current psychosocial stressors to autoimmune disorders, so it pays to ask about psychosocial causality in this case, even as it pays to ask about i.e. sleep apnea in the case of someone who comes in complaining of depression. And when we consider the vast array of interrelated causes and developmental pathways that can be involved in any of these outcomes, it becomes even MORE obvious that the search for disease genes is absurd.
Report comment
Very well said!
Report comment
” Incidentally since I stopped talking to my abusive parents my hashimoto’s antibodies have all but normalized (when I was talking to them I was fast approaching hypothyroidism).”
Hypothyroid patients have low levels of folate and elevated homocysteine. So it makes sense that the stress from dealing with your parents would cause hypothyroid. Stress decreases folate and that can lead to elevated homocysteine and if you have MTHFR mutations you were already predisposed.
But my opinion is obviously biased because I’m a parent.
Report comment
Actually Stephan, the symptoms from different psych conditions can all be caused by LOW VITAMIN D.
In ADHD – learning disabilities, inattention, behavior problems, impulsivity.
In depression – Mood changes accompanied by overwhelming feelings of hopelessness, sadness, loneliness, fatigue, anxiety, loss of interest in activities that previously sparked excitement, suicidal thoughts.
In Addiction – Craving sweets, insomnia, cognitive impairment, liver steatosis. Addiction can also be exacerbated by low Vit D.
In Bipolar disorder – depressed mood, paranoia, psychosis, hallucinations, delusions, low resilience.
In Borderline Personality – Anxiety, sensitivity to stress, anger, mood swings, eating disorders, low oxytocin, decreased serotonin, low dopamine, cutting, impulsivity, cognitive and executive function impairments, inflexibility, obesity, low resilience.
I could go on and on but I think you get the picture. ALL can be caused by low Vitamin D. The lower the level the more severe the symptoms. It would be funny if it wasn’t so tragic. They divided the symptoms into groups and gave them different names and ignored one simple fact…the majority of the population has Vitamin D defficiency….and MTHFR mutations and psych drugs lower Vitamin D.
Report comment
Which psych conditions have low Folate, low Vit D and MTHFR mutations?
All of them. So of course you’re going to see comorbidity. They share many things.
https://www.scielo.br/j/jiems/a/85W3FTYFpKwKKXnFjvGWg3f/?lang=en&fbclid=IwY2xjawLIbJRleHRuA2FlbQIxMQBicmlkETF1ZXlWMDdjODBEUXRkbTIyAR4Tko2SFZ9Xc-tECDHEtxCHqVxw4gq8S6GWzkTJjiAamWA7lxvi381erRTNbA_aem_PEQS9lEx5ku-IqKRfGtTmA
“(Physical pain, though not an official DSM criterion of this disorder, often correlates with it.) ”
Low Folate is frequent in depressed patients and low Folate can cause pain. Low Folate can also affect tetrahydrobiopterin and that increases pain intensity.
“They will gain weight?”
Low folate increases homocysteine which can cause insulin resistance.
Someone once told me that if I started doing research with my mind already made up I would miss much. Best advice I ever got.
Report comment
I think it’s misleading to call it “comorbidity.” It is simply another indicator that none of these categories really mean anything. We should be treating LOW FOLATE (when it’s shown to exist), whether it manifests as depression or anxiety or hallucinations or hyperactivity. “Diseases” should be classified by similarity of cause, not based on superficial manifestations!
Report comment
For sure this is a problem reifying any diagnostic category is going to create this issue but its especially pernicious in psychiatry because they reify their own categories explicitly and dogmatically, and the whole research program gets funded to look for causality in this way (generating in the process an infinite array of weak correlations that are either meaningless or don’t mean what they say they mean). Really though their categories are not very good even as descriptive ones. I try and explain this to some of my friends who are diagnosed, i.e. I am not denying that you feel unfocused and irritable/spacey, unmotivated or extremely despairing, but that does not mean that you HAVE a psychiatric disease that is CAUSING that, and that belief blocks any search for actually meaningful causes. But it’s hard to get that across when the official messaging is so different and I’m not their doctor etc..
Report comment
Precisely.
Report comment
I agree (see also my comment regarding sleep deprivation @Steve).
My more general point would be that even if it’s BIOLOGICAL in the sense that our psychological processes are necessarily embodied, biology (just as the other sciences) simply lack the linguistic means to cover our PSYCHOLOGY.* To me this sounds like a philosophical truism – and that it’s “philosophical” doesn’t make it less true, while in my experience many in biological psychiatry don’t care about theoretical/philosophical insights and naively-positivistically keep producing data. (Recommended read: Turkheimer, 1998, Heritability and Biological Explanation, Psychol. Rev.)
The MiA editors explicitly invited me to write an essay about heritability. If they’d also be interested in my view on why psychology (and psychiatry) cannot generally be reduced to biology (or even physics), I might be able to write this on another occasion.
* Of course, this raises that question about the subject matter of PSYCHOLOGY. Even if I don’t have a final answer, I think that the quality vs. quantity distinction (e.g., our experience, consciousness, shaped by culture) or, as philosophers put it, intentionality and phenomenal content, provide a good answer for the time being. It’d also concede that the boundary between biology and psychology cannot be drawn clearly in all cases. Anyway, agreeing that organisms/individuals cannot be understood without their environment would already help a lot.
Report comment
Chronic vertigo can be caused by high homocysteine.
Report comment