On May 11, the FDA announced approval of the antipsychotic Rexulti (brexpiprazole) to treat agitation associated with Alzheimer’s’ dementia.
Prior to approval, the FDA held a joint advisory committee meeting of the Psychopharmacologic Drugs Advisory Committee and the Peripheral and Central Nervous System Drugs Advisory Committee in April to review the potential approval of Otsuka’s and Lundbeck Pharmaceuticals’ antipsychotic Rexulti for the treatment of agitation associated with Alzheimer’s dementia.
By a vote of 9-1, the FDA Advisory Committee members voted in favor of Rexulti, saying benefits outweighed the increased mortality risk and other known harms of antipsychotics.
However, not everyone agreed. I was the lone NO vote as the Consumer Representative on the Psychopharmacologic Drugs Advisory Committee. In good conscience, I could NOT vote to recommend this additional indication for Rexulti given that the marginal benefit in clinical trials did not outweigh the known side effect profile and higher incidence of deaths.
“I don’t think that the data demonstrated outweighs the dangers of an antipsychotic. I do agree that it is an unmet need, and I hope I’m proven wrong in time. But with this limited amount [of data], I’m not willing to vote yes for this product.” —Kim Witczak , MedPage Today
Antipsychotic Rexulti (Brexpiprazole)
Rexulti was initially approved for treating schizophrenia and major depressive disorder (MDD). In 2005, the FDA issued a black box warning for all atypical antipsychotics after a systematic meta-analysis showed elderly patients with dementia who received antipsychotic treatment experienced a 70% increased risk of death. This led to restrictions placed on nursing homes and a crack down by the government on off-label antipsychotic use in this high-risk population.
Over the years, many of the other manufacturers of antipsychotics have tried to get approval for dementia agitation and failed. Rexulti is the first one to get an official blessing from the FDA for agitation associated with Alzheimer’s dementia. At nearly $12k/month, I am pretty sure Rexulti will not remain the only product in the market for too long. Lundbeck estimates Rexulti could bring in an additional $1B in annual sales.
Treatment for Alzheimer’s disease is big business. In fact, JP Morgan’s Senior Analyst, Chris Schott, stated during the 41st Annual J.P. Morgan Healthcare Conference in early 2023 that Alzheimer’s treatments present significant market opportunities for investors.
“Unmet” Need Has Been Weaponized
“Unmet” need is the new buzz word that allows companies to get priority review or fast tracking at the FDA. It lowers the bar, allowing for less than stellar results and study designs in cases where the FDA can say there is “substantial evidence of effectiveness.” In the presence of some efficacy, the FDA will tolerate a relatively high level of adverse effects, including ones with black box warnings.
It seems many of the new drugs coming before our committee address an “unmet” need. I do not believe this was the original intent of the law or regulations, which allow a little breathing room where there is NO available treatment for a particular condition. However, “unmet” need has been distorted by vested parties saying no current FDA approved product works or that an existing FDA approved product is not 100% effective. Once a product for an “unmet” need” gets FDA approval, the marketing kicks in and the benefits are often overhyped to the healthcare providers and sold as a panacea for desperate families and caregivers.
It kind of reminds me of another “Breakthrough” drug for an “unmet” need for Parkinson’s psychosis. Our committee voted 12-2 and recommended that the FDA approve Nuplazid for the treatment of Parkinson’s disease psychosis based on a six-week study of about 200 patients. Once again, I, along with the patient representative who lives with the disease, were the only two members to vote against its approval. I remember telling the FDA to mark my words, this product would end up killing people. Sure enough, two years after being on the market, the FDA started to receive reports of deaths, and CNN ultimately investigated this product.
Let’s Look at the Rexulti Data
Most of the FDA Advisory Committee members may have been persuaded by what they viewed as “convincing” data with a reasonable safety profile for a high “unmet” need. Yes, agitation from Alzheimer’s is a real issue for patients and caregivers. However, I wonder if the average patient or caregiver will truly understand what the supporting evidence says in relation to the serious harms and potential for early death.
The benefit demonstrated was weak and based on three studies, of which only two reached statistical significance over placebo for the primary endpoint (Reduction of Agitation on CMI Total Score at 12 weeks).
Specifically, in the 1-mg or 2-mg fixed-dose study (study 331-12-283), statistical significance was only reached in the 2-mg group. More importantly, the mean group treatment difference on a score that ranges from 29 to 203 points was just -3.77 (95% confidence interval [CI] -7.38, -0.17, p-value = 0.0404). This amounts to a 2.2% difference that is unlikely to be clinically meaningful and that the FDA itself did not consider “statistically persuasive” during a September 17, 2017 guidance meeting with the company.
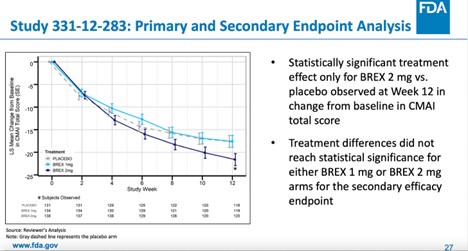
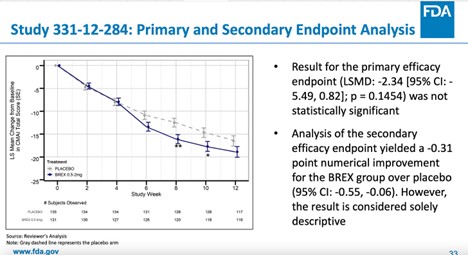
The result of the primary efficacy endpoint (mean change from baseline in CMAI total score at week 12) for study 331-12-284 was not statistically significant.
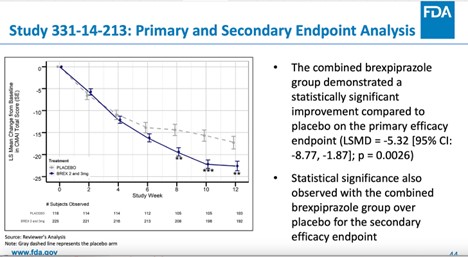
Although a third separate study (331-14-213) found that the combined treatment difference for the 2-mg or 3-mg fixed dose was statistically significant regarding the primary outcome of “agitation inventory” score, an additional analysis on the study showed that for the important secondary endpoint of the “clinical global impression severity” score, the therapeutic effect was distinctively less certain, with only the 3-mg group reaching statistical significance.
In addition, the FDA did not adequately address whether there was a specific population that could be identified in whom the benefits outweigh its risks. There was very little data that shed light on any clear characterization of which patients would benefit from Rexulti. Therefore, the committee could not agree on whether patients with more mild or more severe symptoms were most likely to benefit.
The subjects were relatively young (mean age 74), predominately white (96% of trial participants) and had a low rate of co-morbid psychiatric symptoms (19-26%). There were 123 study sites, with anywhere from 25%-45% (depending on the study) conducted in the United States, and the rest from outside the US. This makes extrapolation to US samples difficult. The FDA should have required a breakdown of how many patients each country provided vs. the rest of the world. It would have been interesting to see if specific sites and/or countries provided the best results and then find out why.
Finally, it was surprising that none of the studies looked at quality of life data for patients. Atypical antipsychotics can have a terrible impact on patients’ ability to function, think, and care about others. I understand that agitation can be very upsetting for caregivers and nursing home staff, but will Rexulti just be another a chemical straight jacket, like other atypical antipsychotics?
What About Safety?
In my opinion, the small benefit in the improvement on the agitation scales stood in stark contrast to the higher risk of death.
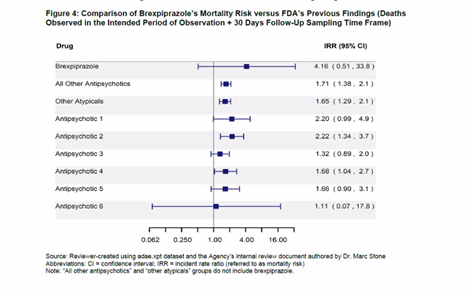
Deaths (Placebo vs. Other Antipsychotics)
Rexulti had a much higher mortality rate than other antipsychotics. In fact, it is more than twice as high a risk than most of the other antipsychotics listed. Also, the study only looked at short-term risk of dying and NOT long-term risk. While the FDA said there were no surprises when they looked at data longer than 12 weeks, that doesn’t mean that the increase in deaths didn’t continue to rise with time. Since this drug is NOT a curative or as-needed drug, one would assume patients could be on this drug for years.
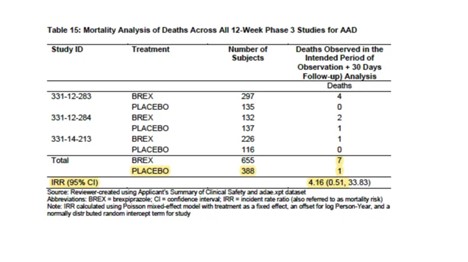
Other Adverse Events of Special Interest (Rexulti, all doses vs. Placebo)
Other common adverse events in subjects treated with Rexulti included akathisia, urinary tract infection, somnolence, and insomnia, and subjects in the treatment arm generally also had a higher incidence of adverse events of “special interest,” such as cardiovascular events.
My FDA Advisory Committee
Since the pandemic, the FDA has operated all their advisory committees virtually. Here’s what my advisory committee meeting looked like over Zoom.
Compare this to what it used to look like before Covid. In my opinion, it is time to get back to in-person meetings or hybrid. There is a level of engagement that is lost over Zoom.

The Voting Question
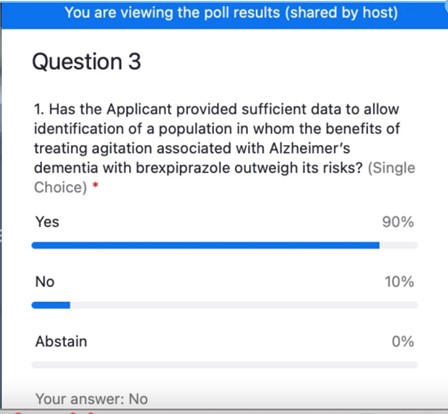

“A glimmer of false hope should never be reason to approve dangerous and ineffective drugs. It doesn’t help patients or their families. So, I guess if I am the only no vote, I can go to bed knowing that I voted my conscience based on the data. I know only too well, what can happen when products get into the real world and millions start taking the product.”
Many times, I leave these Advisory Committee meetings frustrated and wondering why I even bother. However, I have come to realize the importance of my role of reviewing data and asking questions through a different lens. It may not make an immediate difference, but at least I know my comments, questions, and statements will be part of the official FDA record.
Here are a few highlights….
I asked the FDA if any of the other companies had previously tried to get a similar indication in the past. I was quickly shut down and told it was proprietary information and to go look at the Clinicaltrials.gov website, which is for current trials underway. This wasn’t the question I was asking. I was curious about the other current antipsychotics already on the market, like Abilify, Zyprexa, and Seroquel. I am pretty sure Rexulti wasn’t the first drug to be submitted for this indication, given how important the dementia agitation market is.
I also challenged another committee member who said that doctors will do a good job checking in on the patients after 12 weeks to see if the meds are working. I said, “No, that’s not what happens in the real world.” It is more of “set and forget” as one of the members said so cavalierly and then later tried to downplay. Then, you add in the reality of a nursing home environment where a single doctor might be responsible for many patients. Real world patients are NOT being checked frequently like they ideally should be, or advisory members like to think they are.
Since Rexulti is already available to be used off-label, I was curious if any of the practicing clinicians on our committee were using this drug to treat their patients. If not, why not? Neither of the two practicing clinicians used Rexulti, saying it wasn’t their product of choice. Of course, the company’s consulting clinician (and trial investigator) quickly hopped on and said that he has used Rexulti for the past year with great success (N=30). Umm, of course he did. He was on the sponsor’s presentation team and gets paid for his work.
In my closing statement, I told the FDA if they approve this drug for this indication, they had better closely watch the company’s advertising and marketing communications (including industry conferences) on how this drug gets positioned and marketed to the doctors and patients. As we have seen too many times, the benefits are overhyped while the harms are downplayed or dismissed.
Fierce Pharma reported, “The one objecting vote was doled out by consumer representative Kim Witczak, who didn’t think that the data presented outweigh the dangers that are typically present in antipsychotic medications. She also expressed concern over how the drug could be marketed, noting that ‘we need to really keep an eye’ on it.”
Link to FDA Materials — Distributed 2 days in advance of the public meetings.
Link to public comments — The majority are pro-drug and it is always important to consider where they get their funding.
Editor’s Note: This piece was originally published on Kim Witczak’s (UN)Acceptable Collateral Damage Substack.












It is heartbreaking, and morally repugnant, to “treat” anyone – but particularly our children and elderly – with the antipsychotics. Thank you for all you do, Kim, and for your well researched and honest vote against using antipsychotics on our elderly for “agitation” – which, ironically, is largely what akathisia looks like to the “mental health professionals,” despite it being caused by the antipsychotics … so they should know better.
Report comment
Subjective benefits that do not take into account quality of life issues.
Just let that sink in.
Kind of sounds like the reason Mad in America exists.
Report comment
Thanks for doing what you can.
Report comment
According to a recent WSJ article, nearly 80% of the elderly and infirm locked in “care facilities” are prescribed at least one psychotropic drug- when in reality they do not have a real psychiatric illness or diagnosis!
Giving any type of antipsychotic to an elderly person especially if they have medical conditions like diabetes and heart problems is a one way ticket to the GRAVEYARD!
Report comment
Americans are brainwashed to take every pill that their insurance pays for. Tragic. And the kids think a pill will fix their life. Big Pharma won. Mexican Cartel won.
Report comment
My Mom died with dementia. 8 years before her death, while she was in care, and still a mostly coherent – she witnessed another inmate of her facility having fits and thrashing about – unable to communicate their needs.
She said to me: If it comes to this, drug me. I don’t want to be coherent through that. I don’t want to be shamed by it. I want some measure of dignity, even if I’m unconscious.
Something to consider. It surprised me, given my anti-drug stance. But she clearly stated that was what she wanted.
Report comment