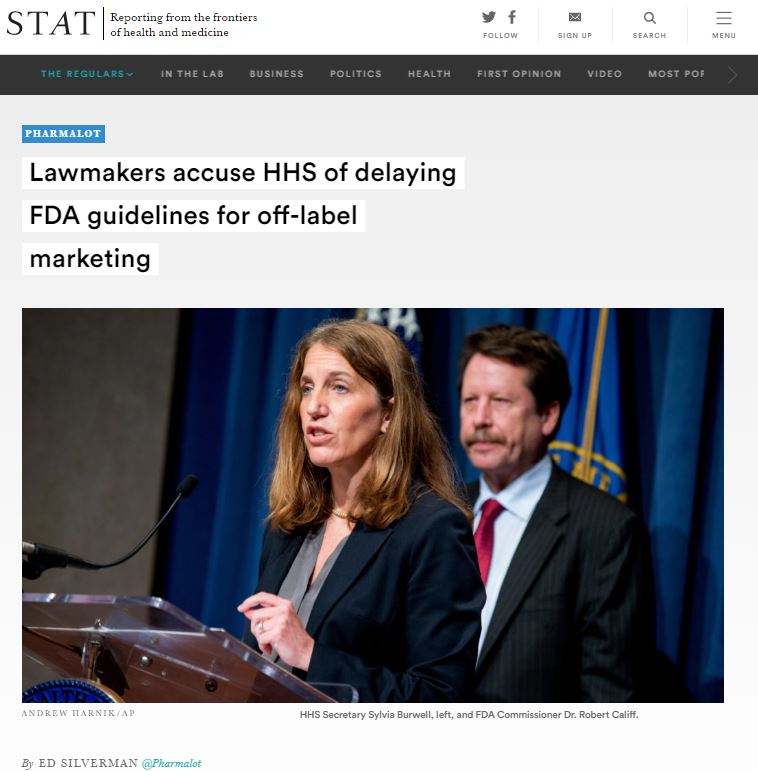
Ed Silverman reports for STAT’s Pharmalot that high-ranking congressmen are accusing the Department of Health and Human Services of deliberately delaying new guidelines on the marketing of drugs off-label, or for unproven uses. In the absence of new guidelines, some worry that court rulings will end up determining policy. Meanwhile, the FDA has expressed concern that marketing claims not backed up by sufficient evidence can lead to public health problems and raise health care costs.