Editor’s Note: Over the next several months, Mad in America is publishing a serialized version of Peter Gøtzsche’s book, Critical Psychiatry Textbook. In this blog, he discusses the misleading statements about depression pills and dosing in the textbooks he reviewed. Each Monday, a new section of the book is published, and all chapters are archived here.
Now that we know that depression pills do not have clinically relevant effects on depression, we may turn to the textbooks. They do not tell us anything of the above.
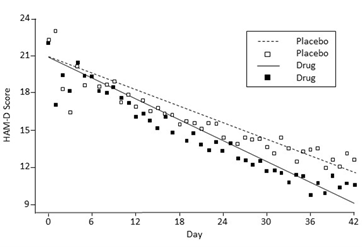
One textbook claimed that one can notice an improvement on fluoxetine already after a few days.19:294 This is utter nonsense. Whether the patients are treated with a depression pill or a placebo, it takes about 3 weeks before they become any better, corresponding to the minimal clinically relevant effect of 5-6 points on the Hamilton Depression Rating scale.273
Another book mentioned that most cases of depression will subside after 2-4 months;17:357 and a third book noted that 60-80% become healthy after 6-10 weeks.18:126 None of the books explained that this is not a pill or a placebo effect but the spontaneous remission of the depression.
The latter book was totally dishonest about the benefits of the pills.18:237 It claimed that psychomotor speed, sleeping pattern, appetite, and mood become normalised, and that depressive thoughts about guilt, inferiority, and suicide vanish. Nothing becomes normal during pill treatment, and the pills double the risk of suicide (as I will discuss further).
This book also noted that it often takes 2-4 weeks before the effects can be observed, not rarely even longer; and that drugs may often improve cognitive deficits, but that this effect often comes after months.18:237 This is like selling snake oil. It doesn’t work but if you wait long enough, you will be better off.
 It is also misleading to claim that, by testing the patient, one can see an effect earlier than the patient subjectively recognises.16:273 The doctor’s assessment on a rating scale is no less subjective, and what the patient feels about the treatment and its unpleasant adverse effects is more important than what the psychiatrist thinks.
It is also misleading to claim that, by testing the patient, one can see an effect earlier than the patient subjectively recognises.16:273 The doctor’s assessment on a rating scale is no less subjective, and what the patient feels about the treatment and its unpleasant adverse effects is more important than what the psychiatrist thinks.
When psychiatrists—rarely—acknowledge that the effect of the pills is small, they often add that this is not important because the patients will benefit from the large placebo effect.
This is a common misconception among doctors and it is due to a logical error. They think the placebo effect is the before-after difference in a group of patients treated with a placebo, which it is not, since spontaneous improvement is also included in this effect.
It is difficult to study the placebo effect because we will need an untreated control group to compare with, and such a trial cannot be blinded. One of my PhD students collected all the randomised trials in all diseases that had included both a placebo group and an untreated group and we found that the placebo effect is generally small, if any.274
One textbook advised that if a 50% reduction in the Hamilton score has not been obtained after 3-4 weeks, the doctor should try to increase the dose, or switch to a drug with another pharmacodynamic profile, and it claimed that this will result in a satisfactory effect in 60-70% of the patients.16:273 Yet again, such statements are highly misleading as the spontaneous improvement is included.
This book noted that a dose-response relationship is poorly elucidated for SSRIs, but claimed that escitalopram was a possible exception, and that SNRIs show a clearer dose-response relationship.16:583 None of this is correct. There are many dose-response studies and they have not shown increased effect with dose (as I will discuss further).
Another book claimed that a dose increase would lead to full or partial remission in 60-80% of the patients and advised that if a tricyclic had not cured the patient, the patient should be admitted to hospital where the dose could be increased to the upper serum level of what is recommended, or even more.18:124
A third book advised to increase to the maximum dose or to switch to a drug from other class.17:360
The psychiatric literature is full of advice and claims like this, which are harmful and contrary to the principles of evidence-based medicine.
We can easily see why it is inappropriate to increase the dose using escitalopram as an example because one of textbooks said escitalopram might be an exception.16:583 The FDA package insert for escitalopram directly contradicts this:275
For adults: “Initial: 10 mg once daily. Recommended: 10 mg once daily. Maximum: 20 mg once daily … No additional benefits seen at 20 mg/day dose.”
The only thing doctors obtain by increasing the dose is increasing the harms. The package insert noted that in two fixed-dose trials, the overall incidence rates of adverse events was 66% on 10 mg and 86% on 20 mg. The incidence of serious harms, e.g. akathisia and deliberate self-harm, also increase with dose,276 and self-reports of violence from patients with no apparent background of violent behaviour are also related to dose.277
Escitalopram is the S-enantiomer of citalopram, the active half of its two stereoisomers, which are mirror images of each other. The tablets exist in three doses, 5, 10, and 20 mg, which are half the doses of citalopram, 10, 20, and 40 mg, as the inactive moiety is not included.
The initial dose of citalopram is 20 mg once daily, which can be increased to a maximum dose of 40 mg/day.278 “Doses above 40 mg/day are not recommended due to the risk of QT prolongation. Additionally, the only study pertinent to dose response for effectiveness did not demonstrate an advantage for the 60 mg/day dose over the 40 mg/day.”278
The package insert for escitalopram mentioned that a crossover dose-response study in 113 healthy subjects showed that the maximum QTcF change compared to placebo was 4.5 ms on 10 mg and 10.7 ms for 30 mg escitalopram given once daily.215 Thus, increasing the dose increases the risk of lethal harms for both drugs.
When the fluoxetine trials X065 and HCJE, submitted to obtain approval for using the drug also in children, were being reviewed by FDA, Eli Lilly submitted a license application for R-fluoxetine, an enantiomer of fluoxetine, which was ultimately withdrawn in part because of QTc interval problems.279 Such problems are an issue with all SSRIs. However, in response to FDA concerns about study HCJE, Lilly argued that the statistically significant increase in mean QTc found with the initial analysis was the product of random variability.280 The FDA’s reviewer responded dryly that, with a P-value of 0.009, the result was, by definition, unlikely to be produced by random variability.
When no additional benefit is seen with the 20 mg/day dose of escitalopram, then the FDA should also warn against using 40 mg/day of citalopram, the corresponding dose of the parent compound, but there is no such warning.278
That no benefit is gained by increasing the citalopram dose from 20 to 40 mg also follows from the shape of the drug’s binding curve to brain receptors. As for other drugs, the relationship between receptor occupancy and dose is hyperbolic (as I will discuss further). At 10 mg, 72% of the serotonin receptors are occupied, which increases to 81% with 20 mg and 86% with 40 mg, not much different from 10 mg.281
There are many dose-response studies of citalopram and other depression pills and they show that increasing the dose does not increase the effect.282-287
In his 2009 book, The Emperor’s New Drugs: Exploding the Antidepressant Myth, psychologist Irving Kirsch explains the fallacy of increasing the dose and why doctors usually do this when their patients do not improve.127:35 The UK Summary of Product Characteristics for citalopram notes that, “In the fixed dose studies there is a flat dose response curve, providing no suggestion of advantage in terms of efficacy for using higher than the recommended doses. However, it is clinical experience that up-titrating the dose might be beneficial for some patients.”288
The summary also has this advice, which comes already on the first page: “The recommended dose is 20 mg daily. In general, improvement in patients starts after one week, but may only become evident from the second week of therapy. As with all antidepressant medicinal products, dosage should be reviewed and adjusted, if necessary, within 3 to 4 weeks of initiation of therapy and thereafter as judged clinically appropriate. Although there may be an increased potential for undesirable effects at higher doses, if after some weeks on the recommended dose insufficient response is seen, some patients may benefit from having their dose increased up to a maximum of 40 mg a day in 20 mg steps according to the patient’s response.”
The summaries for fluoxetine and paroxetine are much the same, with a recommended dose of 20 mg, but the dose can be increased up to 60 mg and 50 mg, respectively.289,290
When doctors increase the dose of depression pills, they are following the manufacturer’s misleading advice, echoed by our spineless and much too industry-friendly drug regulators.2,6,7
It is pure nonsense when the UK drug regulator states that the improvement in patients starts after one week but may only become evident from the second week of therapy. Absolutely nothing becomes evident at any point in time because the improvement, whether the patient receives a drug or not, is gradual (see the graph above). It is therefore also impossible to provide any meaningful assessment after 3-4 weeks to decide if the dosage should be adjusted, but drug regulators abound in such empty advice. When I was young, a common advice was that drugs should only be used in pregnancy with caution. Either you use a drug, or you don’t. You cannot use a drug in pregnancy “with caution.”
It is horrendous that a drug regulator, which is supposed to issue instructions based on solid science, says that, “it is clinical experience that up-titrating the dose might be beneficial for some patients.” Psychiatrists value their clinical experience a lot without realising how misleading it is, but drug regulators should not support them in this illusion. For an individual patient, the clinician has no idea if the patient improved because of an increase in dose, as they have nothing to compare with, but we know from the randomised trials that this is not the case. The text in package inserts comes from the drug companies selling the drugs, which might be the background for the UK drug regulator’s foolish advice.
Kirsch87 mentions a study conducted by German psychiatrists that illustrates these issues.291 Depressed patients who failed to respond to paroxetine or maprotiline were given an increased dose of the drug, following which 72% (65/90) of them improved significantly by showing at least a 50% reduction in the Hamilton score. The catch was that this was a randomised trial, and the dose had only been increased for half of the subjects. Yet the response rate was also 72% (60/83) in the group where the dose was not increased.291
Receptor occupancy for fluoxetine is very similar for 20 mg, 40 mg, and 60 mg.292 Nonetheless, the UK drug regulator advises doctors to double or triple the dose if the response is insufficient.289 The only thing they will get out of this is to increase the harms for no increase in benefit while enriching the industry and its friends, some of whom work in drug agencies.6,7
Harms are very poorly reported in randomised trials, but it is a basic and logical concept in clinical pharmacology that increased doses cause more harm. Harms are often much better reported in cohort studies, at least if the authors or sponsors had no interests in hiding them.
One such study found that the rate of deliberate self-harm among children and adults up to 24 years of age who were new users and initiated high-dose therapy with citalopram, fluoxetine, or sertraline was twice as high, hazard ratio 2.2 (1.6 to 3.0) as among matched patients initiating therapy with usual doses (20, 20, and 50 mg, respectively).276 This is a convincing study because depression severity and suicidal ideation at baseline was similar across the dose categories and because any confounding would need to be implausibly large to nullify the findings.
One textbook noted that, for treatment resistant depression, two depression pills could be combined but warned that there is no evidence that “for sure” supports such treatment.16:275 But we do know for sure that using two drugs instead of one increases the total dose and therefore the harms, for no additional benefit.
The textbooks recommended switching to a drug with another pharmacodynamic profile if the effect is insufficient.16:273,18:123,18:237
One book claimed that treatment resistant depression is seen in 30-40% of the patients within 6-8 weeks;16:275 another book had a lower bet of only 10-20% of the patients.17:364
The first book noted that fewer than 20% of the patients would be treatment resistant if a tricyclic was also tried.16:275 However, this would likely also happen without treatment, as this is the natural course of a depression. If you wait long enough, “treatment resistant” depression disappears in most patients without treatment.
When drugs do not provide meaningful effects, they won’t do so by switching between them. A comprehensive 403-page report prepared by McMaster University Evidence-based Practice Center in Canada concluded that, “There is low strength of evidence evaluating relative differences for any monotherapy or combination therapy approach. All but 2 of 44 studies showed no relative differences in response and remission rates.”285
It was Gordon Guyatt from McMaster University who invented the term “evidence-based medicine,” in 1992.293 He advocated for a new paradigm for medical practice, which “de-emphasizes intuition, unsystematic clinical experience, and pathophysiologic rationale as sufficient grounds for clinical decision making and stresses the examination of evidence from clinical research. Evidence-based medicine requires new skills of the physician, including efficient literature searching and the application of formal rules of evidence evaluating the clinical literature.”
Rigorous assessment of clinical research is a prerequisite for practicing evidence-based medicine, but critical comments about the research that was quoted were almost totally absent in the textbooks. When the authors used literature references, the research was accepted at face value. My studies of psychiatry have taught me two lessons:
- Very few psychiatrists have sufficient understanding of the basics in clinical research and can assess what they read critically. They therefore cannot practice evidence-based medicine.
- Very few psychiatrists read anything at all. They do what their leaders tell them to do who usually do what the industry tells them to do. It is no surprise that psychiatry is a disaster area.
***
To see the list of all references cited, click here.












Very interesting. I have been on anti depressants periodically at my request but also believe that I have seasonal affective disorder. I was offered epilim as an additional mood stabiliser as my diagnosis is bipolar but am happy on my present dosage of quetiapine and mirtazapine. The latter I believe acts as a mild sedative though from past experience I know lower dosages have a profound soporific affect and I do want to function without an afternoon nap which is common among lots of patients on medication for stress disorders. 68 year old
Report comment