In this post for Yale Law School’s CRITical Thinking, Tom Jefferson discusses some of the factors that currently serve to undermine rational and ethical medical decision making.
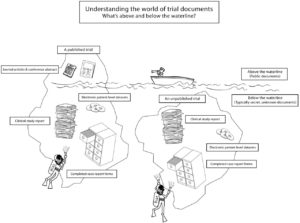
“The evidence base for trials is preponderantly made up of studies sponsored by the pharmaceutical industry for industry purposes (registration and commercialization), but no one seems to have the exact figures on percentage of trials by sponsorship.
By and large non-industrial product development and registration is a thing of the past, although state production of the vaccines persists patchily. Past examples are the state development and testing of vaccines against diphtheria and pertussis.”