Tag: Paxil and sucide
Children Are Vulnerable Cogs in the Psychiatric Machine
My guardian decided to seek out “professional” advice about how to diminish my “outbursts.” I was perceived as a problem that needed to be extinguished into a compliant state.
Dear Scott Gottlieb: Add New Warnings to Paxil Labels
From STAT: Scott Gottlieb, Trump's pick to lead the FDA, should consider pursuing a stronger warning label for the antidepressant Paxil. Paxil's label currently does...
Wendy Dolin Takes on GlaxoSmithKline And Wins — For Now at...
In July of 2010, Stewart Dolin, a partner at the mega law firm Reed Smith, jumped in front of a subway train in Chicago, apparently suffering from akathisia caused by paroxetine. His widow sued, and the jury found GSK negligent in not informing doctors of the suicide risk
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
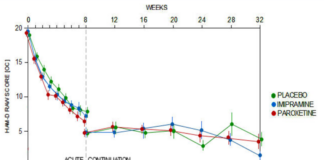
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Is Motivation Worth More Than Expertise?
The strongest evidence we have as to whether a drug causes a problem does not come from RCTs or any other controlled study but rather from good clinical accounts. Even if RCTs were done by angels, so there was no hiding, no miscoding, nothing untoward, RCTs can still hide adverse events. The onus is on large and powerful corporations who have a lot of resources to pinpoint the populations where the benefit is likely to exceed the risk, if they want to continue to make money out of vulnerable people.
Study 329: 50 Shades of Gray
Access to data is more important than access to information about conflicts of interest. It is only when there is access to the data that we can see if interests are conflicting and take that into account. Problems don’t get solved unless someone is motivated for some reason. We need the bias that pharmaceutical companies bring to bear in their defense of a product, along with the bias of those who might have been injured by a treatment. Both of these biases can distort the picture but it’s when people with differing points of view agree on what is right in front of their noses that we can begin to have some confidence about what we have.