
Note from David Healy: It was tempting not to run this post today for fear it might get lost in the wash of the Clinton-Trump debate. But today is the fourteenth anniversary of the day FDA issued an approvable letter for Paxil for children, as well as the fifty-fourth anniversary of the 1962 FDA Act that created the playing field on which Study 329 happened. It’s also World Mental Health Day. Freud might have been amused.
Study 329 recruited its last patient in 1997. The data was analyzed in 1998. Faced with the results, the plan was to pick out the good bits and publish them. This meant never publishing the Continuation Phase – the 24 week extension to the Acute Phase of Study 329. The good bits of the Acute Phase became the Keller et al 2001 paper – possibly now the most famous clinical trial publication of all time.
The Continuation Phase was published for the first time by Le Noury and colleagues in September 2016 – eighteen years later. It is available HERE along with reviews from JAACAP – not the journal in which it was published.
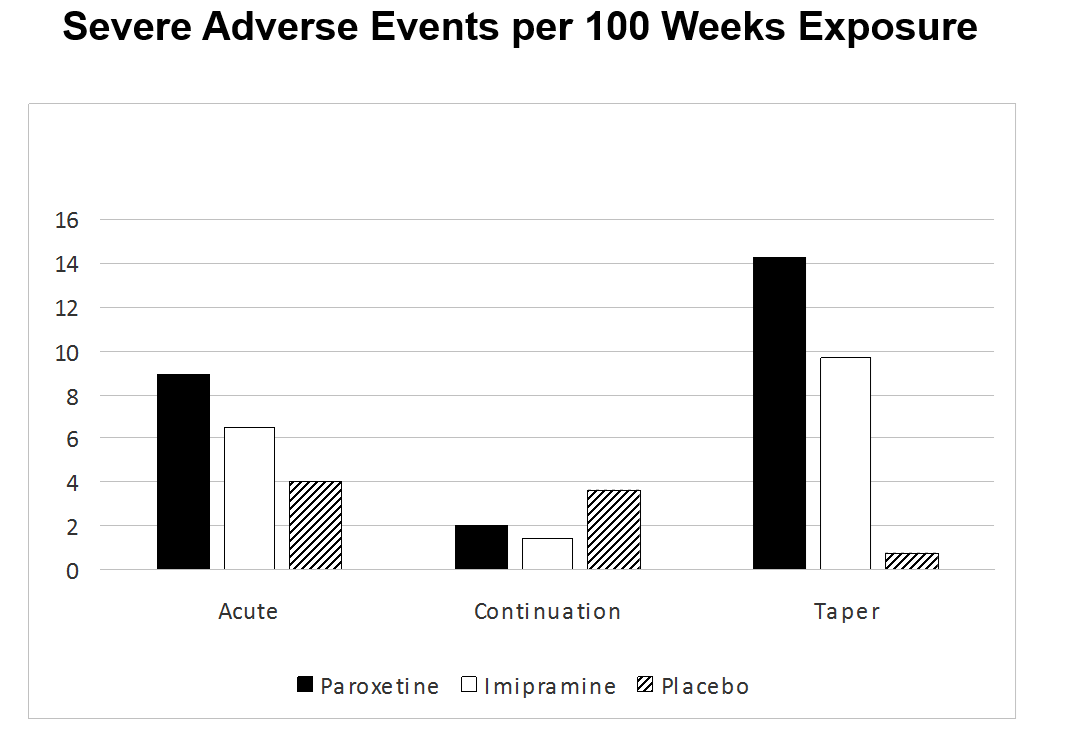
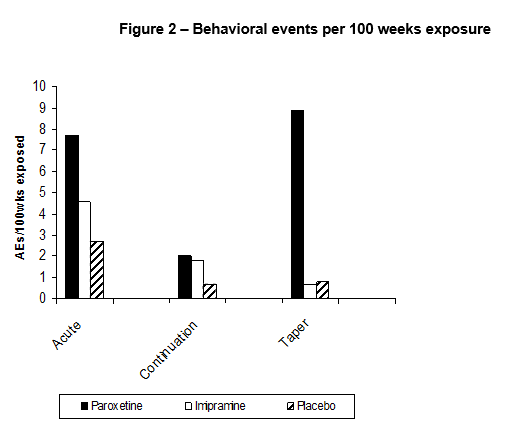
Among the most interesting findings are those from the Taper Phase. Publishing the Acute Phase only meant that the Taper Phase vanished. Whatever way the data are cut, whether in terms of all severe adverse events, or behavioral adverse events or suicidal adverse events the picture remains the same – the taper phase of this trial was the riskiest period for those on active treatment. This will come as no surprise to many who have learnt the hard way after the event. Others like Bruce Springsteen do not yet seem to have put two and two together – see Born to Withdraw.
Or
The years 1997 and 1998 may be as important as the data. The Moneybag image at the top of this page, an internal SmithKline Beecham image from 1997, brings out how the issue was viewed within the company. SmithKline were at the time under intense pressure from Lilly and Prozac, with Lilly running adverts for Prozac like the one below.

The material accompanying adverts like this specifically targeted Paxil — Seroxat — the drug that was Prozac’s most direct competition. This is an unusual thing for a pharmaceutical company to do. They have to be on solid ground. It was also deeply cynical in that Prozac also causes dependence and withdrawal but with the right study its long half life can appear to make this problem disappear.
SmithKline’s response was maybe even more cynical. For women worried about getting pregnant and possible risks to their unborn baby, the short half life of paroxetine and being able to get off it quickly was a blessing. If you were on Prozac, the implicit message was you were “fucked”.
Extraordinarily in the face of this – twenty years ago – most doctors and regulators affected not to notice anything. Were they being adults trying to ignore children squabbling in the back of the car, or were they like terrified kids in Jurassic Park trying not to draw the attention of the Tyrannosaurs sniffing the air around them?
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
It needs an anthem from someone like Bruce Springsteen to find the millions of people whom “Progress” has left washed up on a shore they never wanted to be on. An anthem to put the island on which they have been Shipwrecked on the map.
* * * * *
Adapted from DavidHealy.org















This is surely the most powerful and the most discomforting revelation of the brutal and ruthless tactics of egregious pharma-marketing to date.
Forty years as a doctor, and my naivety in considering drug companies to be ethically based and patient focused is a cause of profound regret.
Thank you RxISK.org and those dedicated to evidence based medicine.
Retired Physician.
Report comment
That is of course, thanks to valid, not fraudulent evidence based medicine.
Report comment
Absolutely, the antidepressants have withdrawal effects:
“Commonly reported symptoms include flu-like symptoms (nausea, vomiting, diarrhea, headaches, sweating), sleep disturbances (insomnia, nightmares, constant sleepiness). Sensory and movement disturbances have also been reported, including imbalance, tremors, vertigo, dizziness, and electric-shock-like experiences in the brain, often described by sufferers as “brain zaps”. Mood disturbances such as dysphoria, anxiety, or agitation are also reported, as are cognitive disturbances such as confusion and hyperarousal.”
And today’s doctors think these symptoms of antidepressant discontinuation syndrome are symptoms of “bipolar.” Even though it states quite clearly in the DSM-IV-TR:
“Manic-like episodes that are clearly caused by somatic antidepressant
treatment (e.g., medication, electroconvulsive therapy, light therapy) should not count
toward a diagnosis of Bipolar I Disorder.”
What a shame today’s mainstream medical community, the psychologists, and the psychiatrists are all so stupid, they can’t even read their own DSM. Plus, they harbor “delusions of grandeur” they “know everything about the meds.” My experience was the only thing today’s psychiatrists know how to do is create “psychosis,” via anticholinergic toxidrome poisoning, while misdiagnosing this as numerous made up and scientifically invalid DSM disorders.
Where’s my malpractice check?
Report comment
“Where’s my malpractice check?”
I think a bunch of us are wondering about this.
Report comment
I think you’re right, Alex. Apparently, the doctors are deadbeats.
Report comment
I often wonder what the people who work for drug companies are like. I’m talking about the upper echelon, the supervisors and movers and shakers and the CEO’s. They know what the effects of their drugs truly are, they know how people’s lives will be destroyed by taking their products, and yet they sit back and put money bag symbols on the information dealing with the drugs. They hide adverse information from studies that have been cooked to make the drugs they want to push look fine and dandy. How cold and ruthless is that?
They create toxic drugs. They send out their drug reps to push these things to uninformed GP’s and to get nursing homes to give things like Haldol to residents who are not as compliant as they should be. They convince an entire nation that everyone is “sick” and “mentally ill” or “depressed” with their direct to consumer advertising. They convince the FDA, which is supposed to protect us, to ok drugs that cause harm. They are manipulative and deceitful and they must be laughing all the way to the bank with their dividends that total in the billions.
How much longer are we going to put up with it?
Report comment