Last week, Robert Gibbons published a paper in the Archives of General Psychiatry in which he claimed, based on his “reanalysis” of the data from studies of fluoxetine in youth, that “treatment with fluoxetine was not found to be related to suicide risk when compared with placebo.” This led Irish psychiatrist David Healy, who has investigated this issue at length, to write a blog in which he categorized the various statistical tricks that Gibbons had employed to come to his conclusion, and he noted that the British Medical Journal called a 2007 paper by Gibbons on this topic “astonishing,” “misleading,” and “reckless.”
But in Healy’s blog, there was a reference to new data from the NIMH’s Treatment for Adolescents with Depression Study (TADS), and therein lies a much more important story.
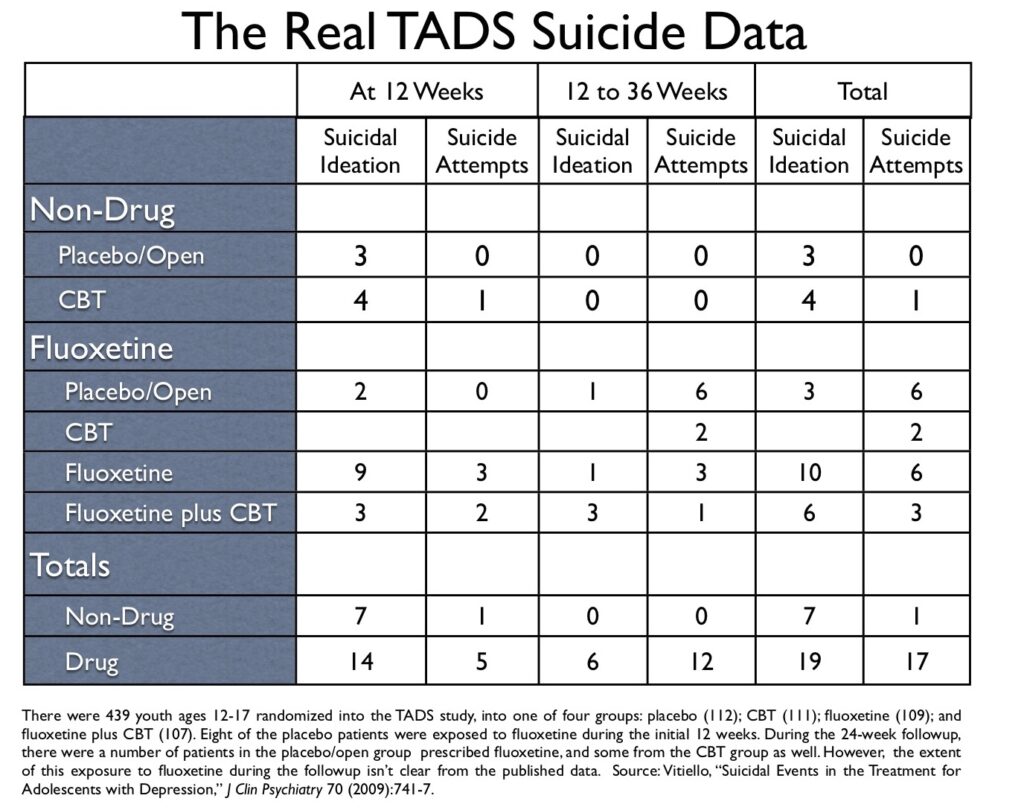
In his blog, Healy published a table on suicidal events from the NIMH’s TADS study of antidepressants in youth, which had been prepared by a Swedish correspondent, Göran Högberg. That table put the suicidal risk associated with fluoxetine in a different light than had been presented in the published articles about the TADS study, and I thus asked Högberg where he had obtained this “updated data.” He pointed me to a 2009 article authored by Benedetto Vitiello, titled “Suicidal Events in the Treatment for Adolescents with Depression Study (TADS),” which was published in the Journal of Clinical Psychiatry. In particular, Högberg pointed me to a table titled “Timing of First Suicidal Event.” And there, hidden in plain sight, was the real suicide data from the TADS study.
The Background to the TADS Study
When the TADS results were first published, the practice of prescribing SSRIs to children was taking an evidence-based beating. First, in 2004 the FDA held a hearing on the increased risk of suicide with SSRIs in children, which led to a black box warning. In addition, the FDA’s Thomas Laughren reported at that meeting that these drugs—with the exception of fluoxetine—didn’t work in children either. Twelve of the 15 pediatric trials that had been conducted prior to that date had failed, as the SSRI had not bested placebo. The FDA, in fact, had rejected the application of six manufacturers seeking approval to sell their SSRIs to children.
Fluoxetine (Prozac) was the one SSRI that the FDA had approved for pediatric use. Two of the three positive studies reviewed by Laughren had come from trials of this drug. But as many critics pointed out, there was no reason to think that fluoxetine was any more effective in children than the other SSRIs. The percentage of children who responded to Prozac in the two positive trials was similar to the response rate in the twelve failed trials; Eli Lilly had simply been better at using biased trial designs to knock down the placebo response rate and thus make it appear that its drug worked. Indeed, Australian investigators who reviewed the trial data wrote in the British Medical Journal that the evidence for fluoxetine’s efficacy in children “is not convincing.” As such, they concluded, “recommending [any antidepressant] as a treatment option, let alone as first line treatment, would be inappropriate.”
The editors at Lancet came to a similar conclusion at that time: The truth, they wrote, was that SSRI antidepressants “were both ineffective and harmful in children.”
Such was the embattled status of SSRIs as a treatment for pediatric depression when the TADS investigators, in 2004, first announced their efficacy results. In the study, 439 youth, ages 12 to 17 years old, were randomized either to placebo, fluoxetine, cognitive behavior therapy (CBT), or a combination of CBT plus fluoxetine. At the end of 12 weeks, the response rate was highest for the combination group (71%), and lowest for the placebo group (35%). Although industry-funded trials hadn’t provided good evidence for the use of SSRIs in youth, this NIMH-funded study had done so, at least for fluoxetine.
At that time, several academic critics, including Jon Jureidini from Australia, noted that the two cognitive therapy groups in the TADS study were unblinded, and that in the only blinded comparison from the study, between fluoxetine and placebo, fluoxetine did not provide a statistically significant advantage over placebo on the primary endpoint, the children’s rating scale. As such, Jureidini concluded that the TADS studied showed “that fluoxetine, like all other antidepressants, is of doubtful clinical importance for children.” But this criticism was mostly ignored, and psychiatry now had its efficacy data for an SSRI in youth.
However, a safety problem did show up in the TADS study. There was an increased risk of suicidal events seen in the fluoxetine group compared to placebo, and the question of that risk hovered for a while over the trial, until it was basically dismissed in subsequent reports. However, with the publication of Vitiello’s 2009 report, and Göran Högberg’s alert reading of that study, we can see how the NIMH-funded investigators finessed this risk, keeping its true scope hidden from the public.
The Suicide Data, Step by Step
The 12-Week Results: Then and Now
In 2006, Graham Emslie and the other TADS’ investigators published the 12-week “safety results” in the Journal of the American Academy of Child and Adolescent Psychiatry. They reported that in the 109 youth treated with fluoxetine, there were 10 suicidal events (9.2%), which was defined as either suicidal ideation or suicidal behavior/attempts. There were three such events in the 112 youth given a placebo (2.7%.) Two in the fluoxetine group attempted suicide, versus none in the placebo group.
Although this data clearly raised a concern, the investigators wrote that suicidal ideation rates had improved in all four groups during the trial, compared to baseline rates, and that nobody in the trial had actually committed suicide. Thus, the excess risk in the fluoxetine group was not seen as unduly alarming.
In Vitiello’s 2009 paper, we find slightly different data. In the 12-week study, there had actually been 12 suicidal events—rather than 10—in the 109 fluoxetine patients. There were three attempted suicides in the fluoxetine group, rather than two. Moreover, in the group of 112 placebo patients, there were 8 that were put on fluoxetine during the 12 weeks, and 2 of these 8 then suffered a suicidal event. But these 2 suicidal events in fluoxetine-treated patients were not included in Emslie’s 2006 report.
Thus, in Vitiello’s paper, we find this additional data: two more suicidal events among the 109 youth randomized to fluoxetine, one additional suicide attempt in the fluoxetine group, and two suicidal events among fluoxetine-treated patients in the placebo group.
The 36-Week Results: The Initial Report by the TADS Team
After the initial 12-week period, the study was unblinded (in the placebo and fluoxetine-only arms), and those in the placebo group who hadn’t done well were offered the choice of choosing one of the three active treatments—fluoxetine, CBT, or a combination of the two—during the 24-week followup.
In 2007, the “TADs Team” published the 36-week results for the three “active treatment” groups, but not for placebo, in the Archives of General Psychiatry. They reported the following “suicidal events” at the end of 36 weeks: 16 of the 109 fluoxetine patients (14.7%) had such an event; 9 of 107 in the CBT-plus-fluoxetine group (8.4%); and 7 of 111 in the CBT-alone group (6.3%).
The researchers did not break down the suicidal events into their two components (suicidal ideation and suicidal attempts), and thus there is no data, in this article, on the number of youth in the study who attempted suicide. And while suicidal events were notably higher in the fluoxetine-only group, the researchers explained it away. They reasoned that CBT likely mitigated suicidal thoughts (as opposed to fluoxetine triggering such thoughts), and thus they concluded in the abstract, “adding CBT to medication enhances the safety of the medication.” (Emphasis added.)
Yet, once again, in Vitiello’s 2009 paper, we find additional suicidal data in the chart titled “Timing of First Suicidal Event,” and when it is included in the data analysis, we see that the risk of suicide, during the followup phase, was found only in fluoxetine-exposed patients. There were no suicidal events in the CBT-alone group who didn’t take fluoxetine during this period. However, some of the patients in the CBT-alone group were in fact exposed to fluoxetine during the 12-week-to-36-week period (we don’t know how many), and there were two suicidal attempts in those fluoxetine-exposed patients.
Here is the bottom line: at the end of 36 weeks, of the 12 suicide attempts in the three groups, 11 were by youth taking fluoxetine.
The TADS Report on the 36-Week Results of the Placebo Group
In March of 2009, the TADS investigators, led by lead author Betsy Kennard, reported the 36-week safety data for all four groups in the American Journal of Psychiatry. As noted earlier, the 112 youth initially randomized to placebo were given the option, at the end of 12 weeks, to opt for one of the three active treatments.
The TADS reported that of the 112 youth assigned to the placebo/open group, 12 (10.7%) had a suicidal event between week 12 and week 36, compared with 32 of the 327 (9.8%) from the three active treatment groups. The researchers wrote that they had also compared suicidal events in the placebo/open group to those taking SSRIs, and the results were the same. There was no excess risk of suicide in those prescribed fluoxetine, compared to the placebo/open group.
In this article, it appeared that 15 of the 112 youth in the placebo/open group had a suicidal event during the 36 weeks (3 in the initial 12 weeks, and 12 in the 24-week followup). It was unclear how many of these events were suicidal ideation and how many involved suicide attempts, as this distinction was not reported. Then, in the discussion part of the paper, the researchers muddied things a bit more, writing that 15 of the 112 in the placebo/open group made “suicide attempts.”
Thus, the impression from this report was that there had been a fair number of suicidal events–and suicide attempts–in youth not exposed to fluoxetine. And having presented the 36-week safety data in this way, the TADS investigators drew this conclusion: Assignment to placebo during the initial treatment period “does not increase harm-related events, including suicidality.” Since placebo didn’t lead to increased suicidality, the use of placebo in research settings was “acceptable,” although “delaying the onset of meaningful treatment in non-research settings is not ethical or clinically appropriate.” Fluoxetine was safe, and in normal clinical care, pediatricians and psychiatrists should prescribe the drug as a first-line therapy (and ideally in combination with CBT.)
Now let’s return to the Vitiello paper and his table, which can tell us the real story about the placebo/open group, from start of the trial to the end of 36 weeks.
Here is the data:
Of the 112 youth randomized to placebo, 103 stayed on placebo during the initial 12 weeks, and three showed suicidal ideation during this period. There were eight in the placebo group who were put on fluoxetine during the initial 12 weeks, and two of these fluoxetine-taking youth developed suicidal ideation.
In the 12-week to 36-week followup, no youth in the placebo/open group who stayed off medication had a suicidal event. However, seven in the placebo/open group who went on fluoxetine suffered a suicidal event, including six who attempted suicide.
During the 36 weeks, no youth randomized into the placebo group attempted suicide while on placebo.
In Vitiello’s table of “first suicidal events,” there are 18 suicide attempts listed for all four groups. Of the 18, 17 occurred in youth on fluoxetine (94%). The one non-drug suicide attempt during the 36 weeks occurred in the CBT alone group, roughly at week five of the study.
In Vitiello’s table, there are 44 total suicidal events listed. Of the 44, 36 were in fluoxetine-treated patients (82%).
Here is a graphic that shows this data.
The Scandal
The TADS study has been used to justify the prescribing of Prozac—and really, by extension—other SSRIs to children and adolescents. The TADS researchers reported that the drug treatment was effective and didn’t increase the risk for suicidal events, as compared to placebo. Adding CBT to medication “enhances the safety of medication,” the TADS researchers wrote.
All the while, the real suicide data was being hidden. The TADS investigators weren’t disclosing the number of suicide attempts, and they weren’t reporting that all but one of the suicide attempts were in fluoxetine-treated youth. Instead, they made it appear that a similar number of suicidal events had been seen in the placebo group, and, at one point, even wrote that 15 in this group had attempted suicide. The real suicide data didn’t appear until Vitiello’s 2009 article, and even then it had to be dug out from a table, which Göran Högberg did.
This, of course, is a hiding of data that puts the lives of children at risk. Maria Bradshaw is blogging on this site, and she has written how her son Toran committed suicide 15 days after being prescribed Prozac. Now imagine if the suicide data from the TADS study had been properly published. Seventeen of the 18 suicide attempts in the study had been in youth on Prozac. Wouldn’t that have served as a warning signal to psychiatrists in New Zealand? Wouldn’t it have served as a warning signal to Maria Bradshaw? Wouldn’t it have served as a warning signal to her son, when he became agitated and aggressive?
And so we can ask: Would Toran be alive today if not for this scandal? And how many other youth have lost their lives in this way, unaware of the real suicide data in the TADS trial?












Thank you again Bob for the strenght and compassion you show. I meet young people every day who use some kind of psychiatric drug, often different so called medication. There are so many children and youngsters being fooled into a situation where they are prescribed drugs and described as individual “problems” instead of being met in a contextual way involving different people and situations.
Young people are told that their difficulties have to do with biological reasons and therefore shall be “treated” with drugs.
Young people come to believe that something is wrong in their brain and they are often told that they have to take their medication otherwise their situation will be much worse.
So are their parents told, that their children need the medication and if they dont take it everything will get worse.
To be continued… When I started to work many years ago therapists, social workesrs and many doctors knew that young people have a way of behaving, feeling and acting which is nothing to be worried about, that is a part of being young. Nowadays it seems as if everything has to be defined, diagnosed and labeled, in a way which has huge consequenses, both for the individual people but also for society and our shared idea about what it means to be a human.
Report comment
Thank you again Bob for breaking down the data to reveal hidden truths about dishonest research that translates into real lives being lost.
This is purely anecdotal, but when Prozac began to be prescribed to children and teens that I was seeing in therapy, I witnessed seversl young clients have dramatic impulse control reactions to the drug.
Over my objections, clinic psychiatrists began recommending Prozac to parents for children and teens I had seen in therapy for several months or more. So, I had a very good sense of their baseline for being able to control the acting out of self-destructive and violent ideation and impulses.
When such dramatic new behavior suddenly appeared, there was no doubt in my mind it was in response to Prozac, and I was often able to get the drug stopped.
My sense was that Prozac can effect sleep patterns in a very negative way so that the person is in a kind of altered state all the time, and also Prozac is itself a powerful agent that can have a dis-inhibiting effect on young people.
I believe Toran would be alive today, and that he isn’t is a tragedy.
When an angry or self destructive emotional state is experienced by developing children and teens, the ideation and impulse that normally could be controlled is often acted out when they are under the influence of Prozac.
It can be tragically fatal for themselves or others if they direct the un-checked emotion of anger outwards.
Everytime I hear of a youth suicide or shooting like at Columbine I wonder if an SSRI was a factor.
Report comment
Evidence has also shown that SSRI’S cause suicide/violence, mania, insomnia and a host of other deadly effects in adults too though this has been covered up and lied/denied by the mental death plutocracy also. Most school and other public shooters were on them including adults.
Once again, I am truly grateful for Robert Whitaker’s thorough, professional, ethical, competent reasearch and reporting exposing the so called biological psychiatrists as the obvious psychopathic malignant narcissists that they are without stooping to their level.
The more such information is available on the web, the more people like Toran’s mother will have access to the information they need to avoid the mental death profession like the plague it is with plenty of evidence to explain why.
Many years ago I was able to save one of my loved ones from this evil menance thanks to the brave work of Dr. Peter Breggin in his excellent books. Although I was attacked for quoting a so called quack, the mental death “experts” involved backed off when I asked for the brain, blood, x-ray and other medical tests that proved so called bipolar while admitting the symptoms for that overlapped with abuse related trauma symptoms. They also backed off on the dangerous meds once I provided Dr. Breggin’s exposure of their lethal effects, not wanting a lawsuit once warned in writing. Of course, in recent times, Dr. Breggin and others joining his courageous campaign to expose the fraud of the medical model of psychiatry/the mental death profession in bed with BIG PHARMA have been more than vindicated.
Somebody said in comments about an article by Robert Whitaker at PSYCHOLOGY TODAY that psychiatrists writing fraudulent attacks on his work were “winning.” Anyone familiar with the mental death profession knows that anyone daring to criticize or challenge them has done so at great cost. Anyone with common sense would know that those who stand to gain financially like psychiatry in be with BIG PHARMA and corrupt politicians and government officials with revolving doors have the most to gain by keeping their fraud status quo going regardless of the cost to their victims while courageous whistle blowers have the most to lose. Yet, the fact that Senator Grassley did many eye opening investigations exposing many of the chief fraud paid shills like “Dr.” child ADHD/biolar Joseph Biederman and “Dr.” Charles Nemeroff, of the mental death profession and that many drug companies have recently been forced by governments to pay milions in damages for fraud for their off label promotion of toxic psych drugs shows that the only way these psychopaths are winning is in the sense of their usual overreaching to win at all costs for greed, profit and status over normal humans that eventually gets them exposed for the monsters they are like the NAZI DOCTORS and their cohorts in crime at their Nuremburg Trials. As courageous experts like Robert Whitaker keep hammering away at the evil and fraud perpetrated by these deranged “experts” or psychopaths by removing their “masks of sanity” (Dr. Hervey Cleckley), the more quickly they will be exposed and removed from power. In the meantime, since trust in the entire broken mainstream medicine pathocracy is at an all time low, those wise people searching for information on the web and elsewhere can save themselves from such crimes against humanity by having access to impeccable, ethical information like that of Robert Whitaker. There are now many excellent articles by experts including many psychiatrists on the web exposing the fraud of the medical model of psychiatry, the junk science DSM and their bogus research or lack therof passed off as science. The more this bogus profession gets pounded by the truth, the less anyone decent will want to be associated with it. I have prayed that this evil will be exposed and it is amazing how much it has been shown to be the usual emperors wearing no clothes even within the last ten years, so there is much hope for its eventual demise. The more that decent people learn the truth, the less they will be willing to tolerate the deadly plague of so called biological psychiatry. Even for those not so concerned about their fellows, as the staggering costs of the fraud treatments of the mental death system to tax payers, health insurance, and its resulting life long disability payments become more clear with our nation’s huge unsustainable debt, more and more people will revolt against this growing psychiatric rape.
Report comment
I really think you could have written this with a lot less words. This reads much like AOAE – painfully redundant. This is coming from your #1 fan.
Report comment
This is a great comment! Leave him alone.
Report comment
Thank you so much for exposing this. Both psychiatrists involved in Toran’s care cited the TAD Study as their basis for believing fluoxetine was safe and effective. I am very angry right now.
Report comment
Bob, can you consider adding a couple of charts to the above article, for easier comparison? Maybe show a bar graph of what the original data suggested, then a parallel bar graph showing the truer picture with the updated data. Or something like that.
Report comment
Thank you, Bob. “agitated and agressive” was exactly my experience after having been prescribed Prozac at age 16. SSRIs ignited in me a cycle of self-harming behaviors which were misdiagnosed as anxiety disorder NOS and bipolar II.
Essentially, the side effects of a single drug (Prozac) prescribed for a situationally appropriate level of sadness paved the way for 15 years of a life on even heavier drugs. Ultimately, I was left numb, sick, cognitively impaired and feeling even more disconnected from my self and others than I had before entering the mental health system.
Thanks to your work, Bob, the bloggers on madinamerica.com and peer-run support networks I have been able to come off of these drugs. Withdrawal has been a challenge, but well worth the discomfort. I’m uncovering the sense of a ‘me’ I’d lost years back. I’m starting to find my way home.
With overwhelming gratitude,
Vanessa
Report comment
Gibbons pretty much wrote his own destiny when he was the co-author of a study that appeared in the American Journal of Psychiatry in 2007 ( http://ajp.psychiatryonline.org/cgi/content/abstract/164/9/1356 )
Gibbons co-authored study claimed there was a correlation between a 22% decrease in SSRI prescriptions and a 14% increase in youth suicide rates between 2003 and 2004, after warnings were issued by the FDA. However, the study was criticised by many.
Respected child psychiatrist ,Jon Jureidini, said the Gibbons study “incorrectly analyzed the relationship between U.S. selective serotonin reuptake inhibitor (SSRI) prescription rates and suicide rates among children.”
“As it turns out,” Dr Jureidini wrote, “preliminary figures are now available from the Centers for Disease Control (CDC), which show that fewer people under age 25 committed suicide in 2005 (when prescribing did decrease) than in 2004.”
“In the year in which suicide rates rose sharply,” he said, “there was no significant drop in SSRI prescribing.”
Despite his critics, Gibbons said at a 2009 Medscape Continuing Medical Education seminar, sponsored by Lexapro and Celexa maker, Forest Labs, “we have seen in 2004 and 2005, the years for which CDC [Centers for Disease Control] has available data on youth suicide rates, the largest increases in youth suicide rates in history since they initially were monitored.”
The disclosure section for the seminar shows Gibbons had served as an expert witness for Zoloft maker, Pfizer, and Wyeth Pharmaceuticals, maker of the antidepressants, Effexor and Pristiq.
Gibbons is a Professor of Biostatistics and Psychiatry and Director of the Center for Health Statistics at the [UIC] University of Illinois at Chicago College of Medicine, according to his bio on the Department of Psychiatry’s webpage.
He is also a witness for GlaxoSmithKline in the current UK Paxil litigation, of all things he is their “expert statistician” witness.
Definition of expert is – A person with a high degree of skill in or knowledge of a certain subject.
It’s apparent that Gibbons does not fall into this definition and his stance regarding youth suicide from antidepressants from the SSRi family of drugs just echoes that of his paymasters.
On a footnote, you highlighted the death of Toran Henry, the journey to seek the truth regarding his suicide has been a battle for his mom, Maria Bradshaw. A shameful inquest that can only be likened to a game of bullying against Maria by all those involved in Toran’s death.
It should not take a mother or father of a bereaved child a journey of heartache to get to the truth. The truth should have been published and not suppressed purely for profit.
Someone lied and ended Toran’s life prematurely. Manslaughter or murder? It’s one of the two and the likes of Gibbons should be held accountable for publishing misleading information.
Way I see it, Gibbons stance on antidepressant medication should carry a public health warning and those responsible for prescribing Toran Prozac should, at the very least, be struck off the register [even though the psych involved was not even on the register] and be made to answer the overwhelming evidence that has existed some time about Prozac and other SSRi’s.
One child’s death should never be used as a “benefit/risk” ratio, particularly when the benefit of taking these drugs is akin to taking a sugar pill.
The more people who speak out for the banning of these drugs, the better. To say they “do help some” is one of the psychological reasons why doctor’s still prescribe them.
They don’t work and they can cause premature death and/or horrific withdrawal problems. Big pharma have spun this to their advantage and will continue to do so because they have never really been held accountable, those settlements in US courts are not payments for the suffering and loss, they are to, once again, suppress information sought in disclosure…and so the cycle continues.
Bob Fiddaman
Author of ‘The evidence, however, is clear…the Seroxat scandal.
Report comment
Bob, thank you once again for the painstaking and vigilant way you attend to data and what it actually reveals. So many of us take these scientific “findings” at face value, given our busy lives and the faith all of us have been taught to have in the scientific method.
Unfortunately, as your work has shown, this blind faith has created a crack through which so many precious lives have fallen. A fault line so deep and long that it has created shaky ground in all developed nations.
Maria, I wish these risks were more widely known and that your son was still with you today. Thank you for trying to help other families and children move safely through suffering. You are, most certainly, a mother bear.
Report comment
The bottom line is the stats being used for suicide reporting are unimpressive and inaccurate considering the children are killing themselves and their deaths are not being reported as suicides, but accidents. So really the numbers mean nothing, when your don’t count them correctly. Example, the 20/20 piece on the drugging of foster care children. see video on http://www.ablechild.org. Gabriel Meyers. The treating psychiatrist punishment was he cannot participate in medicaid program as a provider anymore. Gabriel Meyers not even worthy to report his death as a suicide. It was reported as an accident. Gabriel hung himself with a shower hose at the age of seven. The death should have been ruled suicide induced murder with charges against the treating psychiatrist. Until we start counting these wasteful human deaths correctly, we can’t validate the data to say the least.
Report comment
I know this data can seem confusing. I’ve added a link in the post to a graphic that summarizes the actual suicide data from the TADS study, as drawn from the table in Vitiello’s 2009 report, which listed individual suicidal events and whether the youth were on fluoxetine at the time.
Robert Whitaker
Report comment
Thanks for following the chain of custody, so to speak, of the data, Bob.
Is there a body at NIMH where a request for review of findings can be submitted? If not there should be.
This stuff shouldn’t be fought out in blogs.
Report comment
This direct TADS NIMH data MUST be made VERY available available and well known to the scientific community. Decisions are made DAILY throughout the Western world to place children on fluoxetine and other SSRI’s as a direct result of the intitial fraudulent data. Child psychiatrists cannot know of the real data without such HIGH LEVEL PUBLICATION of these incredibly important findings.
With the greatest gratitude for your work.
Report comment
PRINCIPAL TADS INVESTIGATOR DR JOHN S MARCH CRITICAL LINKS TO ELI LILLY, MANUFACTURERS OF FLUOXETINE – AND FAILURES TO DISCLOSE:
2006 paper: “The Treatment for Adolescents With Depression Study (TADS): Methods and Message at 12 Weeks” JAACAP 2006 March, Silva, Vitiello, TADS team – Correspondence to Dr. John March –
Disclosure: Dr. March is a consultant or scientific advisor to Pfizer, Eli Lilly, Wyeth, GlaxoSmithKline, Jazz, and MedAvante; he is a stockholder in MedAvante; he is on the speakers’ bureaus of Pfizer and Eli Lilly; and he receives research support from Eli Lilly, Pfizer, and Wyeth. Dr. Silva is a consultant for Pfizer. Dr. Vitiello has no financial relationships to disclose.
2007 paper: “The Treatment for Adolescents With Depression Study (TADS)Long-term Effectiveness and Safety Outcomes.” Arch Gen Psych, 2007 TADS team. Correspondence: John S. March, MD. Submitted for Publication: August 28, 2006; final revision received December 23, 2006; accepted December 26, 2006.
Financial Disclosure: Dr Findling has received … Dr Posner has received … NOTHING re Dr March
Funding/Support: The TADS is supported by contract RFP-NIH-NIMH 98-DS-0008 from the NIMH to Duke University Medical Center (principal investigator, John S. March, MD, MPH). Eli Lilly and Company provided fluoxetine and matching placebo under an independent educational grant to Duke University.
AT THE TIME OF SUBMISSION, MARCH WAS A CONSULTANT OR ADVISOR TO, AND ON THE SPEAKERS BUREAU FOR ELI LILLY AND OTHERS. AND DID NOT DISCLOSE.
2009 Journal Clinical Psychiatry paper. “Suicidal Events in the Treatment for Adolescents with Depression Study (TADS)” John March is a consultant or scientific advisor to Pfizer, Lilly, Wyeth, GSK, Jazz, and MedAvante and holds stock in MedAvante; he receives research support from Lilly and study drug for an NIMH-funded study from Lilly and Pfizer
Dr March also lied in 2012 paper “Drug Development in Pediatric Psychiatry: Current Status, Future Trends.” Child and Adolescent Psychiatry and Mental Health 2012 in stating – Dr. March has not engaged in industry promotional work, e.g., speakers bureau or training, for over 15 years.
Some simple maths – that’s a lie. See 2006 paper disclosures.
Dr Marsh remains co-chair of NAMHC’s Neurodevelopment Workgroup Roster – a key advisory body to the NIMH – and must surely be removed forthwith for this egregious dishonesty, and his publications where his enormous undisclosed COI have occurred, eg TADS, should also surely be retracted from the literature.
Dr John S March KEY NIMH POSITIONS presently:
NAMHC’s Neurodevelopment Workgroup Roster
http://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/namhc-workgroups/namhcs-neurodevelopment-workgroup-roster.shtml
AND A REPORT http://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/neurodevelopment_workgroup_report.pdf
Scandalous. Deserving of high profile publicity and retraction of positions and papers.
Report comment
Dear Dr. Whitaker,
When I first read this most recent Gibbons et al. study (“Suicidal Thoughts and Behavior with Antidepressant Treatment,” Archives of General Psychiatry, published online February 6, 2012) exonerating SSRIs insofar as the promotion of suicidality in children and adolescents is concerned, I was troubled (and perplexed) by the authors’ conclusion. In fact, the authors go so far as to raise concerns about the ligitimacy of the “Black Box Warning” for SSRIs prescribed for children and adolescents.
I am grateful to you for your fastidious and informed analysis of the Gibbons et al. article, as well as your scholarship with regard to the extant TADS-related data. You, Dr. Göran Högberg, and Dr. Benedetto Vitiello are to be commended for your due diligence. I hope that you plan to submit a “Letter to the Editor” of the Archives of General Psychiatry in order to call essential attention to the flaws in the Gibbons et al. article while it is fresh in the minds of the professional (and lay) readership. This matter deserves to be exposed to aggressive and informed discourse.
With sincere gratitude,
David Creasey
Report comment
If I understand this correctly, it seems almost criminal of the researchers to hide these findings. (I wonder if criminal charges are plausible.) Bob, I don’t mean to critique your writing (and thank you for the added chart!). But I wonder if for the argument to have the most impact, there ALSO needs to be a way to present it as concisely as possible. In case this is helpful, below is my initial take on a more concise version, which should of course be checked for accuracy, or rewritten however needed:
NIMH’s Treatment for Adolescents with Depression Study (TADS) reported that the rate of suicidal-related events for the two “placebo groups” versus the two “antidepressant groups” was roughly the same: 44% of events in the “placebo groups” versus 56% of events in the “antidepressants groups”. A conclusion from this was that antidepressants don’t increase risk of suicidality in adolescents.
But the data as presented are dangerous flawed, because:
(1) After the first 12 weeks of this 36-week study, those in the so-called “placebo group” were offered the choice of antidepressants.
(2) Even during the first 12 weeks, a few youths in the “placebo group” were exposed to anti-depressants.
This confounds the meaning of the so-called “placebo groups” and “antidepressant groups”. It ends up that 82% of the suicidal-related events occurred in patients given antidepressants, only 18% of events in patients not on antidepressants.
Report comment
This issue will only be settled in the law courts. Are there any plans?
Report comment