Tag: British Medical Journal
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
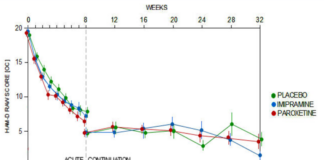
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Study 329: Conflicts of Interest
The BMJ states that it takes on average eight weeks from submission of an article to publication. The review process for Restoring Study 329 took a year, with a three-month review process involving six reviewers to begin with, and then a further four reviews in a four-month process, leading to a provisional acceptance in March that was withdrawn.
Here’s the Real Data: No Increase in Suicide Attempts Following Black...
A British Medical Journal study led by Harvard Medical School's Christine Lu suggested that black box warnings about increased suicidality in youth who take antidepressants actually led to increases in adolescent suicide attempts. However, the latest in a stream of critics of that conclusion are the authors of one of the key studies cited by Lu in support of her team's analysis.