Tag: study retraction
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
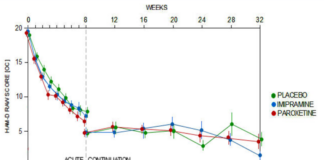
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Study 329: Conflicts of Interest
The BMJ states that it takes on average eight weeks from submission of an article to publication. The review process for Restoring Study 329 took a year, with a three-month review process involving six reviewers to begin with, and then a further four reviews in a four-month process, leading to a provisional acceptance in March that was withdrawn.
Study 329: By the Standards of the Time
The controversy over “Study 329” on the effects of Paxil in teen depression has raised questions about the state of ALL medical research. I decided to look at the research for the most recent psychiatric drug approved by the FDA, a new antipsychotic called cariprazine or Vraylar. I located twenty studies of Vraylar on www.ClinicalTrials.gov, the U.S. government-sponsored registry for clinical trials. Three were still in process, and seventeen were completed. Not one had shared its results on the government website, a supposedly mandatory step.
Study 329: MK, HK, SK and GSK
It is appropriate to hold a company or doctors who may be aiming to make money out of vulnerable people to a high standard when it comes to efficacy, but for those interested to advance the treatment of patients with any medical condition it is not appropriate to deny the likely existence of harms on the basis of a failure to reach a significance threshold that the very process of conducting an RCT will mean cannot be met, as investigators' attention is systematically diverted elsewhere.
Psychiatry’s Thalidomide Moment
The authors of Study 329 began recruiting adolescents for a comparative study of Paxil, imipramine and placebo in 1994 and finished their investigations in 1997. They dropped a large number of their original cohort, so the randomness element in the study must be open to question. Late in 1998, SmithKline Beecham, the marketers of Paxil, acknowledged in an internal document that the study had shown that Paxil didn’t work for adolescents in terms of the two primary and six secondary outcomes they had established at the start of the study. In a nutshell, Study 329 was negative for efficacy and positive for harm, contrary to their succinct upbeat conclusion.