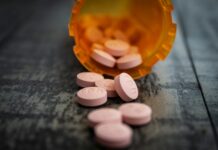
In January 2017, Bill H.3594 An Act Relative to Benzodiazepines and Non-Benzodiazepine Hypnotics was filed by Representative Paul McMurty in the Massachusetts State House. Benzodiazepines are tranquilizers which include Ativan, lorazepam, clonazepam, Klonopin, Xanax, alprazolam, Restoril, Valium, and many others. Non-benzodiazepine hypnotics are ‘Z sleep drugs’ like Ambien, Lunesta, and Sonata. This legislation would require practitioners to obtain written informed consent regarding risks associated with long-term use, dependency and addiction. It would also mandate warning labels concerning long-term use.
The Bill received 23 co-sponsors in early April. This is a promising number considering it was filed at the beginning of a two-year legislative session along with nearly 6,000 other bills. It will be heard by The Joint Committee on Mental Health, Substance Use and Recovery.
This proposed legislation is an amended version of a bill which ‘went to study’ following a hearing in April of last year. No hearing date has been announced yet for H. 3594. The bill was seasonably filed this time which means that it must be heard by The Committee by the end of this legislative session. There is a possibility it could be held in as long as a year or more. However, we do not know when in the session it will be called, and notice is usually as short as a week (see Stay Connected below).
What are the specifics of the Bill?
- Practitioners must obtain patients’ written informed consent regarding information on misuse and abuse by adults and children, risk of dependency and addiction and risks associated with long-term use.
- Pharmacists must ensure that prescription labels include a bolded cautionary statement concerning risks associated with long-term use.
- The Department of Public Health will produce pamphlets to be distributed by pharmacists which contain educational information on misuse and abuse by children and adults, risk of dependency and addiction, storage and disposal, addiction treatment resources and a telephone helpline operated by the Bureau of Substance Abuse Services.
- Written prescriptions for less than a 10-day supply will not be refilled.
- A special commission of nine members will study safe discontinuation protocols to minimize withdrawal symptoms. They must report back to the legislature within four months. The commission will be made up of legislators, a psychiatrist, the secretary of health and human services, the commissioners of public health and mental health (or their designees), representatives from the Bureau of Substance Abuse, Center for Addiction Medicine at Massachusetts General Hospital and the “addiction treatment community.” (This is a change from the original version which called for The Department of Public Health to establish and enforce specific discontinuation regulations.)
Why is this Bill Important?
The history of Benzodiazepines has been described as a 60-year scandal. (Keep in mind that they relate closely in many effects to the Z sleep drugs which this proposed legislation has grouped them together with.) The makers of Ativan themselves write that this class of drug in general should not be prescribed for longer than 2-4 weeks1. They note that withdrawal symptoms like rebound insomnia can appear upon discontinuation after as little as one week of use. Within such short periods of time, the question of benefits outweighing risks can become irrelevant because the original pathologies the drugs were initially aimed at may worsen compared to their baseline presentations due to compensatory adaptations in the central nervous system. In other words, the drugs have a tendency to make things worse.
There are usually myriad additional problems unrelated to the initially targeted symptoms as well. This happens at ‘safe, small’ doses taken as prescribed. In a Xanax study2 conducted for the FDA, manufacturer Upjohn noted that patients became addicted after eight weeks of use and at that point, panic symptoms increased 350% upon withdrawal. Patients and prescribers often incorrectly view cumulative negative effects of long-term benzodiazepine use as underlying or unrelated medical or mental pathology. A common story is patients being lead to further unnecessary and sometimes detrimental treatments.
Evidence3 of compensatory changes can be seen in the likely upwards of 15% of long-term users who develop a severe protracted or post-acute withdrawal syndrome (otherwise known as PAWs) after discontinuing these drugs. The often tortuous and debilitating syndrome can last months to years after cessation. Benzodiazepines have been linked to Alzheimer’s, brain shrinkage similar to that seen in alcohol abuse, and 30% of fatal opiate overdoses. And despite being on The Beers List for medication potentially inappropriate for older adults, the drugs remain widely and consistently prescribed to the elderly throughout the world.
There has been some impressive organizing over the decades concerning benzodiazepines, yet little has changed. In 1979, Sen. Ted Kennedy led a senate subcommittee hearing into the drug’s’ dangers. The 1980’s and early 90’s saw the largest class action suit ever against British drug manufacturers which was unfortunately abandoned, largely due to legal costs. In 2002, the Maine Benzo Study Group comprised of physicians and other healthcare professionals concluded there was no evidence for long-term use4. One recent positive development was that in 2016, the states of New York and Pennsylvania issued ‘prescribing guidelines’ recommending against use longer than several weeks. Nevertheless, benzos’s sordid history regarding long-term use is not translating to practice or common knowledge: they remain some of the most widely prescribed drugs in the world5.
How You Can Help
1. Submit Testimony: Email your or your loved one’s personal story of benzodiazepine harm to Attorney Garrett Burns at [email protected]. Your testimony will be presented as evidence to the Joint Committee as they decide whether or not the bill moves forward. The sooner you can send your personal story the better, since it is unclear when the committee will meet. You do not need to live in Massachusetts to send in your story. You can send your story from anywhere in the U.S. and internationally, there are no restrictions.
Please include the following information in your email:
Full Name
Town and State (if you live within the U.S.)
Country (if you live outside of the U.S.)
Use “Bill H.3594” as the subject line
If you are writing from outside of Massachusetts, include how this bill will affect your state/country. For example, if this bill passes in Massachusetts, you are hoping a similar bill will pass in your own state/country.
Your testimony should be addressed: Dear Chairs and Members of the Committee on Mental Health and Substance Abuse
Keep in mind: the symptoms you were originally prescribed for, what you were prescribed, how long you were on the drug or drugs and the impacts they had on your/your loved one’s life. Finish with how this legislation could have changed your situation.
Unless you are a medical or mental specialist, it is not important to focus on things like what benzodiazepines are or how they work: we want to avoid redundancy. Avoid spending time on the theoretical and stick to what has personally happened to you.
2. Contact MA Legislators: Tell them you support Bill H.3594 and why. This can be done at any time. State senators can be found here, representatives can be found here. MA residents especially have weight with their local representatives. In the week prior to a hearing, it would be helpful to contact local representatives as there is hope that a letter of support will circulate the State House.
Stay Connected
- Follow Facebook Page: “Massachusetts Bill H.3594: Informed Consent for Benzodiazepines & Z Drugs”
- For MA residents only: provide me your email at [email protected] and local address (which you will also have to submit for testimony) and request to be notified of the hearing via email
- Keep an eye out for a possible announcement on MIA
Let’s finally do this.














I became the worst benzo ‘addict’ ever I was up to 14mg of Xanax a day at its worst.
I did not know how addiction worked I always thought that drug addicts fell in love with the high and just said screw everything else in life. I was all wrong a benzo buzz is not that great what drives addiction is the withdrawal, the most nasty horrific nasty thing and the only way to make it stop is to take more. I did not know about that part.
Like most of us that lived it as tolerance withdrawal took hold, that part where tolerance goes up faster then the prescribed dose leaving you in a constant state of withdrawals I took more then prescribed to get relief from this nasty predicament they of course then accused me of “abusing” it.
Blame the victim is the name of the game, cause next came the hello my name is ______ and I am a addict part [my fault] in substance “abuse” treatment.
I remember in group saying things like Hello my name is _____ and I was a victim of the crooked psychiatric pharmaceutical industry, I was not doing that “I am an addict” my fault thing.
They are the masters of victim blaming and most people fall for it.
What is interesting is after getting informed consent the hard way, learning myself how drug dependency really works I can take a few benzos once in a wile for a total day off from anxiety. Now I know don’t take it to the point of dependency and I will be fine.
Don’t call the drug warriors on me but occasionally someone will divert a few pills my way. I would never take them every day again I know what happens now. Want to take one quickly turns to need to take one.
Informed consent would save alot of people, just explain how the need to take more to avoid withdrawals part really works, I detail. Some people will still think “it won’t happen to me” but its better then now where people find out the hard way.
Report comment
The full film
https://vimeo.com/188181193
Sure would like some legislators to see it…
Report comment
When are the politicians going to leave medical issues to the Doctors. You don’t see any Doctors trying to be politicians do you. Our government is already deep into our lives as it is, and it’s only going to get worse. Some people can’t use benzo’s because they become addicted, but you can’t write a blanket law based on the minority of people who abuse their medications and are drug addicts. I’m disabled due to a Neurosurgeon cutting a nerve in my spine, which left me in a wheelchair for 3 years. I developed a rare nerve disease called RSD/CRPS and it’s more painful than Cancer. I’ve had to fight Insurance companies and pharmacies, just to get the pain medicine that my Pain Management Doctor prescribed for me so that I can get out of bed and function somewhat. I do not get high on my pain meds, or benzos because people like me who suffer from chronic pain, take their meds to take the edge off of their pain, and to help them function or get through the day and have somewhat of a quality of life. Politicians like John Kasich of Ohio just wrote a new law that only allows Doctors and Dentists to prescribe only a 7 day supply of pain meds for their patients. Kasich is clueless because everyone is different, and he can’t write laws regarding writing pain medications for 7 days, based on all of the heroin addicts who being lumped together with people in pain with serious ailments. The politicians are the ones who are perpetuating this conspiracy linking heroin addicts to normal people who happen to be taking pain meds for some kind of injury or illness, and how to take care of patients who suffer from many painful diseases. How many Doctors to you see that are trying to be governors of a state. 0 Doctors, because they have a lot more common sense than John Kasich, who is clueless idiot. I’ve been taking 3 valium a day for 20 years for muscle spasms in my spine, because I’ve had 4 failed back surgeries, and I’ve never taken more pills than I’m prescribed, and I always have pills left over at the end of the month. The same goes for my pain meds. I’m glad that I go to a Pain Management Doctor who knows what he is doing, because he treats me with respect and dignity unlike career politicians like John Kasich who are trying to make a name for themselves and are totally clueless on what it’s like being in severe pain 24/7. I wish John Kasich could live one day in my shoes, so that he could feel the chronic severe nerve pain that I go through on a daily basis 24/7. Maybe then he wouldn’t write ridiculous laws about prescribing pain medications for a 7 day period. I think it’s about time that advocates like The U.S. Pain Foundation, People with Pain Matter, and Uniting Pain Warriors take a stand and educate these idiot and clueless politicians who are writing blanket laws that affect everyone who takes pain medications and benzodiazepines. The United States is becoming just like Germany was in the 1930’S, because our government is in every one of our lives, and spying on us with the NSA under Obama’s approval, which recently just came to light, and it is totally illegal. I don’t know about anyone else but I’m tired of big government taking our freedoms and liberties away from us a little bit at a time. One day we will wake up and soldiers from the U.N. will be knocking on our doors, and telling us to come with them. Some people who will read my post might think I’m crazy, and if you do just google how our government has bugged our computers, microwaves, ovens, I-phones, cable T.V.’s, and any way else the NSA can sneak into our lives and record every word that we are saying. It’s time that Americans take a stand about how our own government is treating it’s citizens, and because of 911 Homeland Security has a free pass and can use any type of surveillance equipment to track our every move, and who we associate with and what we say. Our government has the means to spy on any American that they want to, at any time they want to just because they can. WAKE UP AMERICA! Thank God for medical marijuana in Ohio!
Report comment
Jeff,
Being diagnosed with cancer, I was hoping to get a prescription for medical marijuana because it seems it multitasks as far as dealing with symptoms. My bleeping state doesn’t allow it which greatly angers me. And by the way, the state legislature is controlled by Republicans so this is not a Democrat vs. Republican issue.
Report comment
Jeff Do you approve of this ?
Typically, consent forms used in many helping interventions (medical or otherwise) serve to protect professionals, not inform and empower clients so they can make intelligent choices about their fate or well-being. If it seeks to achieve the latter goal, we believe that informed consent for psychiatric drug treatment should contain the following elements: a statement to the effect that the biomedical status of what is to be treated is uncertain, even speculative; unbiased, up-to-date information concerning treatment options, including of course strictly psychological forms of treatment; realistic and comprehensible information concerning somatic and psychological effects of drug use and drug withdrawal, both in the short run and in the long run.
We are inclined to think that no one who was so informed would consent. Of course, this is an exceedingly complex question. It would be greatly simplified if clinical psychiatry and clinical psychopharmacology rested on rigorous research and valid findings, showed genuine concern for the patient’s or subject’s bests interests, and operated in a mental health system designed to meet patients’ or clients’ needs. We have argued elsewhere in detail that this is not the case (Cohen, 1997; Jacobs, 1995; McCubbin & Cohen, 1996). If our analysis has any validity, no consent form will do. Nevertheless, therapists, clinicians, or researchers must obviously make a sincere effort to convey the risks, the drawbacks, the unknowns of psychiatric drug treament (even if many prospective patients might understandably recoil).
A MODEL CONSENT FORM FOR PSYCHIATRIC DRUG TREATMENT
I, the undersigned, understand that I am about to be prescribed one or more drugs by Dr. __________________.
The drug(s) I am to be prescribed is (are) the following:
____________________.
____________________.
____________________.
____________________.
I understand that a DSM-IV diagnostic label has been assigned to me, based on my doctor’s (and perhaps also on other people’s) subjective judgment of my speech, manner, and behavior during our meeting, which lasted approximately _____ minutes. I am aware that I will never be able to remove this diagnosis, or any other that will be added in the future, from my medical record.
I understand that although my doctor says that I am sick or that I have a treatable illness or disease, he or she is just using a figure of speech and cannot establish, with any test or procedure known to medical science that I in fact “have” the “illness” implied by the diagnostic label.
Indeed, I am aware that although medical opinion may now hold that a “chemical imbalance,” a “brain abnormality,” or some physical problem “underlies” or “produces” my distress or suffering, no objective information (through lab tests, scans, etc.) concerning the state of my body has been obtained in order to arrive at a DSM-IV diagnosis. If, by chance, such information has been obtained for that purpose, I understand that this information plays no role whatsoever in fulfilling any criteria for any DSM-IV diagnosisor diagnoses that I have been given by my physician except perhaps for diagnoses related to drug-induced disorders such as tardive dyskinesia.
I have been informed that the drug or drugs which my doctor is prescribing cannot cure whatever “illness” or “chemical imbalance” medical opinion might believe I have but can only affect symptoms of my distress or suffering.
I understand that the drug I am about to take cannot restore any of my physical or psychological functions “back to normal.” Rather, the drug is expected to produce many new mental and physical symptoms, which might help make my original complaints seem less disturbing for a while.
I understand that it is exceedingly difficult to determine what is brought about (both desired and unwanted) by a psychoactive drug which has wide and diverse effects on the brain and other organ systems. I further understand that the problem of how to accomplish this adequately is a controversial issue within psychiatry and the Food and Drug Administration (FDA).
I realize that FDA approval of the drug I am about to take is based upon very short-term studies (usually 6 to 8 weeks) which are designed, paid for, and supervised by the drug’s manufacturer. I further realize that the FDA does not require or expect that a drug’s full range of adverse effects will be known prior to marketing and prior to lengthy exposure of ordinary patients to that drug.
I am also aware that the FDA’s knowledge about the drug’s adverse effects after marketing comes mostly from spontaneous physician reports, the FDA itself recognizes that these reports are just “the tip of the iceberg” of the probable true frequency of adverse effects. I know that wording in the package insert and in the Physician’s Desk Reference is the outcome of a complex negotiation between the manufacturer and the FDA. I also realize that it sometimes occurs that the FDA belatedly learns that the manufacturer has not fully disclosed to the FDA what it actually knows about a drugs’s adverse effects. Finally, I understand that despite FDA approval for psychiatric drugs being granted on the basis of short-term studies, the longer-range consequences of continuing druf use are not systematically studied by any responsible organization or government agency.
If I am consenting to take the drug as part of a research study, I understand that the researcher’s primary interest and loyalty is not to me as a patient and not to my personal interests or welfare. I understand that the “needs of the research project” come before and have priority over my own personal needs.
I understand that the drug will have a wide range of effects on my brain, body, consciousness, emotions, and actions. My sleep, my memory, my judgment, my coordination, my stamina, my sexuality are likely to be affected.
I understand in particular that the effects of a psychoactive drug may undermine my ability to accurately monitor and report upon just how the drug has affected me, even impaired me, perhaps in a dangerous direction (judgment, social perception, impulse control, etc.). I further understand that what to do to protect me, as a patient or subject, against this possibility is a basically unanswered problem in psychiatric drug treatment and research.
I understand that effects that have a 1 in a 100 chance of occurring are actually considered “frequent” effects that should be mentioned to an adult, competent, prospective patient like myself. My doctor (or the researcher) has specifically advised me that the following toxic or adverse reactions may occur, and has provided these estimates of the frequency of their occurrence in patients like me: __________________________________
__________________________________.
I understand that I may experience an adverse effect which might then disappear after a few days or weeks. This disappearance will usually mean that my body has developed a tolerance to the drug’s presence, not that the effect will never bother me again in the future.
I understand that if I inform my doctor of the occurrence of adverse effects, he or she will have five basic options: (a) cease the drug, (b) decrease the dose, (c) increase the dose, (d) switch to another drug, or (e) add another drug. I understand that no rules exist to determine which option is best to follow in individual cases, and it is likely that several options will be followed simultaneously. I also understand that most doctors are not likely to report to the FDA any adverse effect they suspect or have observed, contributing to the generally inadequate picture of a drug’s true impact on patients like me.
I have been informed, if I am prescribed a neuroleptic drug such as Haldol or Risperdal and if I take it regularly for a few years, that I have at least a 30% chance over the next 5 years of developing tardive dyskinesia, a possibly irreversible disorder characterized by abnormal involuntary movements of my face or other body parts. I have been informed that I may also suffer from other acute or chronic movement problems, such as parkinsonism, akathisia, and dystonia, and their associated unpleasant mental states.
I have been informed, if I am prescribed a tranquillizer like Xanax or Klonopin and I take it regularly for more than three or four weeks, that I run the risk of becoming physically dependent on it. I will then have a good chance of experiencing “rebound” insomnia and anxiety, and many other unpleasant sensations, when I try stopping the drug, or even while I continue to take it. I understand that these drugs are not effective antianxiety or sleep-inducing agents after a few weeks of use. I realize that some people are unable to withdraw and must therefore permanently endure the consequences of daily use.
I have been informed that if I am prescribed lithium, I do not have a “lack” of lithium in my body, nor can such a “lack” be demonstrated by any existing test. I understand that the blood tests that I will undergo regularly will be for the sole purpose of determining just how much lithium has been introduced into my bloodstream and whether this could produce toxic symptoms, since, as a result of the mental dullness that lithium is expected to produce, I will be in no position to recognize some of these toxic symptoms.
I understand that the drug is likely to provoke various unpleasant effects when I stop taking it, especially if I stop too suddenly. I understand that although withdrawal reactions are systematically ignored in psychiatric drug treatment or research, they might represent the worst part of my whole drug-taking episode. I further understand that these reactions will often closely resemble the original symptoms for which the drug was prescribed to me, and are likely to be taken for a return of these symptoms (a “relapse”), rather than for withdrawal effects. I realize that my doctor or the researcher is likely to interpret these reactions as a sign that my “illness” is chronic and that my drug is “effective.”
I also understand that once I have been taking drugs for months or years, I will have much difficulty to find a health professional to assist me in withdrawing prudently and safely from the drugs, if I so wish.
Having understood the above, I realize that the drug treatment may cause severe pain or discomfort, worsen my existing problem significantly, or even damage me permanently. However, most doctors or experts will never formally or informally acknowledge that the drug harmed to me in this manner. I will have practically no chance of proving that the drug caused my damage and obtaining compensation.
I understand that no body of research clearly shows that the problems my diagnosis or diagnoses require or respond more favorably to drug treatment than to one or more forms of nondrug treatment. It is obvious to me that nondrug treatment would enable me to completely avoid whatever dangers or risks are associated with taking the drug or drugs I am agreeing to take. My doctor (or the researcher) has made it clear to me that existing evidence does not indicate that it is in my best interest to choose drug treatment as a first recourse.
I am choosing to be treated with (write in the name of the drug or drugs) for the following reasons: (provide ample space; this section must be filled in by the patient or subject):
________________________________________________________________
________________________________________________________________
________________________________________________________________
Signed: __________________________________.
The original is here http://laingsociety.org/colloquia/polofdiagnosis/modelconsent.htm
Report comment
Hello again The Cat,
I just finished reading you post about informed consent, and I agree with you on psyche drugs like paxil, effexor, and many other serotonin reuptake, inhibitors ( I hope that I spelled every word correctly). When I was prescribed Paxil I felt like committing suicide because the side effects of Paxil, made me feel really spaced out and I thought I was losing my mind. Like I mentioned in my first post, I take valium for very severe muscle spasms in my back, and legs and valium is the only muscle relaxer that has ever helped me. I’ve tried every muscle relaxer on the market, and that’s why my Doctors have prescribed me valium for years, it works. I haven’t taken any other psyche drugs so I can’t comment on those, and I’m not sure where I stand when it comes to informed consent for taking valium for muscular reasons, rather than for psyche reasons. Valium is a class 4 drug and I respectfully disagree with you on the side effects that happen when you stop taking it. I know this firsthand because I only take 2 10 mgs. of valium a day, and I weaned myself off of them a few years back and I had no side effects whatsoever. Under a physician’s care that’s the proper way to quit using benzo’s, is to wean you down for a couple of months so that the patient has little or no side effects at all. Because I only take 2 a day I’m sure the side effects would be minimal to none if I considered to stop using them. I do agree with informed consent on the psyche drugs that I mentioned above because in my opinion they cause more damage than valium could ever do, and many many many people have committed suicide because of the side effects of drugs like Paxil, and Effexor. I truly believe that medical marijuana is the answer to all of my prayers, because I would love to stop taking all of the pain medications and even the valium, because medical marijuana has the capability of a great pain reliever for nerve pain, and also it relaxes you, so I wouldn’t need valium anymore. I live in Cincinnati, Ohio and I could go up to toledo right now and purchase a medical card, and then travel to Ann Arbor Michigan and purchase anything that I needed and it’s all legal.
Report comment
I find this part of it really applied in my situation: “I understand that the drug I am about to take cannot restore any of my physical or psychological functions “back to normal.” Rather, the drug is expected to produce many new mental and physical symptoms, which might help make my original complaints seem less disturbing for a while.”
When I was first diagnosed with schizophrenia I was hearing voices and had delusional thinking that was so extreme that I ended up being hospitalized. My guess is that the severity of the hallucinations and delusions would have diminished on their own without any drugs anyways, but unfortunately, they forced me to take drugs, and what I noticed is that my original symptoms of schizophrenia paled in comparison to the side effect symptoms of the meds: akathisia and tardive dyskinesia.
Report comment
Jeff is correct and when politicians make these laws they are practicing medicine without a license. They kill people in drug court that way all the time. https://psmag.com/news/american-judges-are-playing-doctorand-doing-harm
The odds overwhelmingly predict when you get the politicians involved they will only make things worse for people. Decades of the drug war confirms this.
From Above “Written prescriptions for less than a 10-day supply will not be refilled.”
That would have landed me in the ER or in a bad part of town looking for to buy them that way or the liqueur store cause alcohol is the only available anti anxiety drug available over the counter.
What happens to all the people who get anxiety attacks and panic disorder when “Written prescriptions for less than a 10-day supply will not be refilled.” ??
Alcohol , street drugs and I would imagine going to the Emergency Room will result in more psych lockups and off label prescribing of chemical nightmares like Olanzapine/Zyprexa, poisons that make a clonopin habit seem minor in comparison.
You have to do the math on the whole thing, what happens when you make it a bitch for doctors to prescribe benzos ? The simple math says great less people live the Benzodiazepine dependency nightmare BUT how many more people are now subject to Paxil , Effexor, Cymbalta , Zyprexa ? Four of many nasty “non addictive” drugs that also produce dependency and wicked protracted withdrawal syndromes. Zyprexa withdrawal makes kicking Clonopin look easy.
“A special commission of nine members will study safe discontinuation protocols to minimize withdrawal symptoms.” and the “addiction treatment community.”
Thats me the addiction treatment community but I know how they operate Paxil , Effexor, Cymbalta , Zyprexa …. I do my best to confront it but that is what they do is look for psych labels for insurance purposes and push more pills.
From addict to psych patient- Dual diagnosis is what we call them. https://www.madinamerica.com/2017/05/alcoholism-is-it-a-disease/
I know damn well the safe discontinuation protocols and all this will lead to more psych lockups and Paxil , Effexor, Cymbalta , Zyprexa, Depakote, Lithuim , Trileptal…. How could a “special commission” of those nine members lead to anything else ?
I bet “big pharma” would like a war on benzos too, who wants to sell generic Clonopin when the lowest GoodRx price for the most common version of Latuda is around $1,055.00, 14% off the average retail price of $1,227.66 ??
I am all for Informed consent thats why I pasted the whole thing above instead of just a link that most people won’t click on but like Jeff said politicians playing doctor is evil, the odds overwhelmingly predict more harm then good.
Report comment
Great points Cat that I hadn’t considered about the benzos! Thank you for posting them.
You and I were already in agreement regarding the prescription of pain pills.
Report comment
Hi The Cat,
To expound on “What happens to all the people who get anxiety attacks and panic disorder when “Written prescriptions for less than a 10-day supply will not be refilled.” — They get a prescription for over 10 days. I myself am not totally clear on the rational behind that point let me say. I have to imagine it is in part aimed at curbing abuse. If people are tapering or taking the drugs as prescribed, then they do not have to worry about running out.
Also, the purpose of the commission is to explore safe tapering guidelines (in large response to people suffering from being taken off too quickly).
I hope you consider sending in your story!
Report comment
Jeff,
You needn’t worry about a proposed law concerning better informed consent for benzodiazepines affecting your access to opiates or even benzos.
I wonder about your reaction, or really non reaction, to the section “Why is this Bill Important”. You speak about people suffering in connection to abuse or addiction. As the post mentions, there have been many,many thousands of people who have taken this class of drug as prescribed and suffered dearly. Here is a quick video compilation of people testifying to this. https://www.youtube.com/watch?v=SMzaxAo-sxI
Because it may not have happened to you personally, does not mean that it does not happen. See your own example of taking Paxil. People have a right to know the whole truth in order to make truly informed decisions. That is what this Bill aims at. You say that doctors do not try to be politicians. Those forces most certainly do exert influence on public policy. Moreover, those interests cannot beheld responsible for policing themselves; we need a system of checks and balances which represents the most important stakeholder here: the public.
I would also really encourage you to explore the points about originally targeted symptoms.
Thanks for commenting,
Sonja
Report comment
First of all, the courageous woman responsible for this bill isn’t a career politician, she is a victim of the psychiatric system. You would know that if you bothered to read her biography. Second of all, this bill isn’t about banning benzodiazepines, it’s about informed consent. Maybe actually read the article before commenting next time.
Report comment
Sorry if that was too harsh.
Report comment
There are MDs that are politicians! Disgraced former Congressman Tim Murphy, a Psychologist, who spearheaded the draconian HR 2646 Mental Health Bill, as well as the awful Steve Gottlieb, an MD, who heads up the ever-so-corrupt FDA, just to name a couple.
Report comment
Jeff,
As one of the few people on this site who feels there has been an unfair crusade against people on pain medications, I mostly agree with your post.
However, I do need you to clarify this statement:
“”Some people can’t use benzo’s because they become addicted, but you can’t write a blanket law based on the minority of people who abuse their medications and are drug addicts. “”
If you are talking about people who took benzos, many folks take them as prescribed and still become addicted and have a horrible time getting off the meds. They were not given fully informed consent and that is what the above bill is about.
I am so sorry about your situation. After experiencing pain from a dental extraction and not taking my meds quickly enough, I couldn’t imagine what it must be like like to live with it 24 hours a day.
Report comment
Addiction is the wrong word for most who get caught in the trap.
Benzos changes the brain. Iatrogenic damage from as little as a week of use can happen. It’s BRAIN DAMAGE.
The ADDICTION language is harmful to the millions of us who are going thru hell years later after we got off the ‘medication’ (AS PRESCRIBED!!!) .
Watch the film and educate yourself cuz otherwise you are not really grasping the issue here.
ps
not at all against pain meds…true informed consent is rare when it comes to benzos and antidepressants, however
I would have never have taken either if I had known the rest of my life would be filled with so much impairment and loss…BRAIN DAMAGE!!!!
Report comment
Thank you The _cat for backing me up. I totally agree with everything that you said. When will the people of the United States open their eyes, and see for themselves what politicians are doing to us. Free speech is being taking away from us by powerful lobbyists in Congress, and it seems like only protestors/rioters are the only people who have freedom of speech. Laws are being written every day that affect us without our knowledge, just like this archaic law regarding Benzodiazepines. Big Government is ruining our once great country, and The United States is becoming more and more like a communist country, because our freedoms and our liberties are being taken away from us little by little. Benzodiazepines are not dangerous drugs, and that’s why they are listed as class 4 drugs, which means that there is a low level of dependence when using theses drugs correctly. The only people that these drugs harm are the drug addicts, who Doctor shop and buy them on the streets for a cheap high. I hope that this bill stays in Massachusetts and does not continue on to the rest of our county. Why doesn’t big pharma have a law against Tylenol because it does way more damage to the liver than the actual drug that it’s mixed with. The reason why is because this makes too much sense and that is the reason why big pharma continues to make this awful dangerous drugs. Keep up the good fight The_Cat, and do not ever trust our government, because they are only in this so called “drug war” for the money and they don’t give a s##t about the average citizen of the United States. Big Pharma and the Insurance company’s run the world, so beware of what they do and what they say because it’s all one big lie. Thank you again The_cat for being informed and not letting our government pull the wool over our eyes. Have a good day and God Bless you.
Report comment
“Benzos are not dangerous drugs”
Watch even the first 10 minutes of the film linked to above. Dr. Heather Ashton (the only doc to have ever researched how to get off benzos safely and the fallout from their use) compares what’s happening to people all over the world as a result from iatrogenic damage taking benzos *as prescribed* comparable to the thalidomide scandal.
Report comment
Thank You AA! I really appreciate you comments. People can become dependent on benzos as well as pain pills, but there is a difference between becoming dependent and being addicting to the medications. Addicts take more than they are prescribed and people who become dependent on their medications take them as prescribed, but their bodies become dependent on having a certain amount of what ever medication they are taking.
Report comment
Thanks for the reminder HB and I apologize for using it in my response to Jeff. I am assuming your post was addressed to him but I still wish I hadn’t used the word.
Report comment
Jeff@59, The_Cat, and AA
Many of the things you are saying here are supporting potentially harmful and unscientific beliefs regarding both the benzodiazepine AND the opiate crisis in the world today.
Jeff you said:
“Benzodiazepines are not dangerous drugs, and that’s why they are listed as class 4 drugs, which means that there is a low level of dependence when using theses drugs correctly. The only people these drugs harm are the drug addicts…”
Nothing could be further from the truth. As we speak, benzodiazepines are harming literally millions of people around the world. With rare exceptions, people who take this category of drug for longer that two weeks (including time for withdrawal) are bound to get in some type of trouble or experience serious iatrogenic damage.
While there are some people who use these drugs in an addictive manner and yes, benzos are intimately connected to the drug (especially with opiates) overdose crisis, THE VAST MAJORITY OF PEOPLE SUFFERING FROM BENZODIAZEPINE USE ARE TAKING THEM “AS PRESCRIBED.”
Jeff, we don’t know all the specifics of your medical issues, but it is improper to extrapolate a whole series of medical and scientific points from your own particular personal experience, and then condemn others if it somehow it does not exactly match your own subjective experience.
There is NO WAR ON CHRONIC PAIN PATIENTS IN THIS COUNTRY! Chronic pain pain patients are a small minority part of a much larger group of people who have been harmed by Big Pharma and the medical establishment because of a long history of unsafe and unscientific prescribing of several different categories of drugs.
We have no way of knowing (over many years) what would have happened to chronic pain patients if they had been treated with several different types of carefully directed medical and rehabilitative protocols BEFORE ALLOWING THEM TO BECOME PHYSICALLY AND PSYCHOLOGICALLY DEPENDENT ON OPIATES AND SOMETIMES ALSO BENZOS.
Once these drugs have been used for any significant period of time, it is extremely difficult to reverse course and go back to other medical protocols that could be potentially MORE effective. NO, these chronic pain patients SHOULD NOT BE RIPPED OFF THEIR CURRENT COCKTAIL OF PRESCRIBED DRUGS. As particular victims of the harmful prescribing patterns of organized medicine, they are entitled to the highest level of care and attention in order to solve (on an individual basis) a way to manage their pain.
Unlimited or unrestricted access to benzos and opiates IS NOT the solution to these problems. Right Wing phrases that point the finger at “big government” being the problem are misleading at best, and they let the real criminal here off the hook.
It IS NOT the SIZE of government that is the identified problem here, it is, rather, WHAT CLASS OF PEOPLE IS GOVERNMENT MADE UP OF, AND WHO DO THEY SERVE?
Richard
Report comment
“Ayn Rand, Rand Paul and Paul Ryan walk into a bar. The bartender serves them tainted alcohol because there are no regulations. They die”.
Report comment
Good one.
😀
Report comment
I’m definitely stealing that one.
Report comment
Richard
I beg to differ in regards to the ‘war on pain patients’.
My neighbor, who passed a month ago (unrelated to story), went cold turkey off her Effexor and trazadone rather than submit to a pee-test that the clinic now requires for all patients receiving triplicate rxs.
I think people in pain should be able to access pain meds, but supervision and follow up are needed…and that rarely happens here. An accurate diagnosis would be good, too, but again…
BTW cat, I like your consent form above. That’s worth saving for future use.
Report comment
Hi Humanbeing
I know that there is now much more scrutiny of the drugs prescribed to chronic pain patients. And as it typically happens in this System, the victims of this long history of harmful prescribing are subjected to more prejudice to take people’s focus away from the real criminals. But the use of the “war” analogy is far too extreme and overstates what is actually happening here.
Richard
Report comment