Since I was fifteen years old and heard about antidepressants for the first time, I have disbelieved the concept of antidepressants and other psychiatric drugs. Therefore I felt very honored when the opportunity of doing a PhD on harm from use of psychiatric drugs at the Nordic Cochrane Centre with Peter C. Gøtzsche became a reality.
Up until now I have primarily worked with animal studies, investigating what animal research could teach us about harm from exposure to psychiatric drugs.
I have learned three things from working with animal studies:
- The studies are methodologically poor or poorly reported, or both.
- There is little behavioural research on long-term harms from previous exposure to drugs.
- Despite the poor quality of animal studies we still use them as the gateway to doing human trials.
Despite these challenges to animal research, we found that mammals exposed to psychiatric drugs do suffer from long-term harms. The mammals showed impaired sexual behavior (antidepressants, primarily SSRIs), impaired locomotion (neuroleptics), impaired cognition, learning and memory (neuroleptics), depression (SSRIs), and impaired fertility (ADHD drugs). The findings from the three systematic reviews that we undertook are discussed in more detail below.
We wanted to investigate harms from exposure to different categories of psychiatric drugs in mammals. The methods we used were inspired by the Cochrane Handbook and a risk of bias tool for animal studies. We based our systematic reviews on controlled studies, as described in the predefined protocols.
Our main criteria were that all mammals must be treatment-naïve and no behaviour or trait must be primed by operation, genetic modifications or other drug treatments.
A short summary of each systematic review is presented here.
Long-term changes in observed behaviour after exposure to psychiatric drugs
We wanted to investigate long-term harms from exposure to any approved psychiatric drugs with a subsequent drug-free follow-up period of at least 90 days (link) to find out if differences between the intervention and control groups persisted after end of exposure.
We included 33 studies: 16 studies used antidepressants, nine used neuroleptics, six used benzodiazepines and two used ADHD drugs. Despite the fact that 27 studies tested more than one drug (but only one drug per animal), this is a small number of studies on long-term harms. This is clearly an area highly under-researched. Fifteen of the 33 studies subjected the animals to more than one behavioural test.
Twenty-seven of the 33 studies were undertaken with rats, whereas the rest used monkeys, hamsters, cats or mice. For the 27 included studies the median treatment length was 14 days (range 6-365 days) and the median drug-free follow-up period was 150 days (range 90-365 days). In nine studies the intervention was prenatal. We found that animal research is highly heterogeneous and thus challenging to combine and that the studies did not resemble the clinical situation as the intervention period was short.
No studies investigated outcomes related to pain or aggression. There were no differences in sleep patterns (three studies from the same research group) or addiction (one study). The outcome dimension anxiety comprised 25 different outcomes measured in eight different tests with a variety of drugs — therefore no firm conclusion could be made based on these results that varied both in favour of the control and the intervention groups. Our findings generally showed harms to be persistent for social (including sexual) behaviour; movements; cognition, memory and learning; and depression.
We found that the animals lost interest in sexual activities when previously exposed to antidepressants, just as was concluded from the findings in another review from our group (link, see the short summary below). We saw that the six outcomes for sexual behaviour were in favour of the control group, and for number of mounts and mount latency significantly so.
For depression the results were diverse. However, when looking at SSRIs only (three studies) the results for immobility as a measure of human anhedonic behaviour were significantly in favour of the control group.
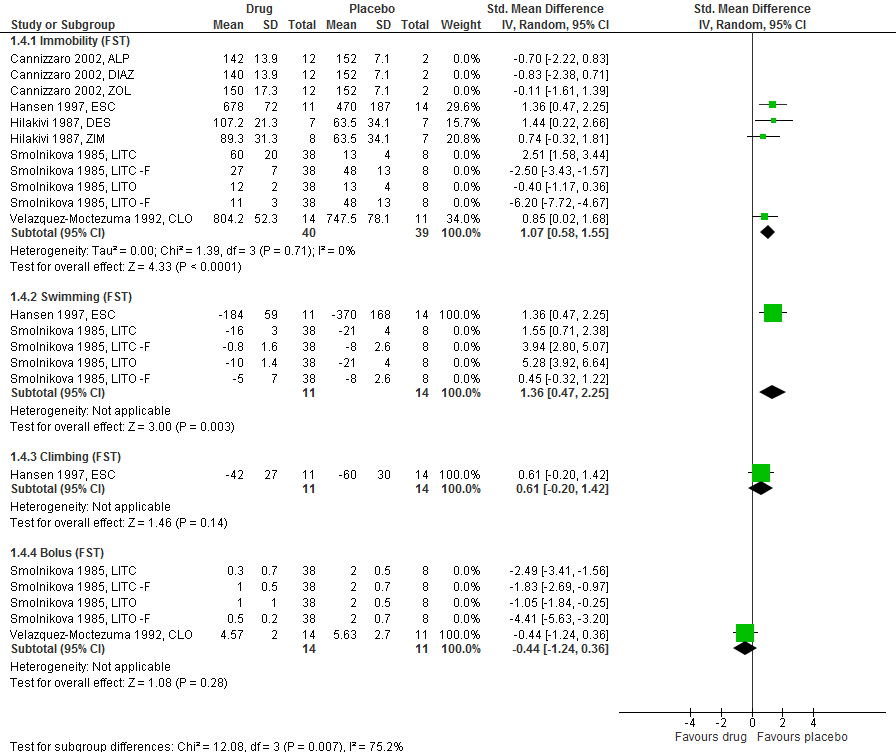
For impact on movements, neuroleptics caused vacuous chewing movements in rodents after a drug-free period (six studies). The chewing movements are involuntary, as if the rodents are chewing on food when there is none. These movements are comparable to tardive dyskinesia.
For the dimension cognition, memory and learning showed that exposure to olanzapine decreased learning in one of three tests while exposure to diazepam impaired memory and learning significantly in eight of nine outcomes.
In conclusion, we found that animals do experience long-term harms from previous exposure to psychiatric drugs. However, research in this area is clearly lacking as the evidence base on long-term harms from use of psychiatric drugs is extremely sparse, both in animals and humans.
Persistent sexual dysfunction after early exposure to SSRIs
We included 14 studies, published between 2006 to 2013, which assessed whether the use of SSRIs can lead to persistent sexual dysfunction after a drug-free period of any length (link). Four studies used prenatal intervention; all studies were with rodents.
For all outcomes suitable for meta-analysis — mounting behaviour, intromission behaviour and ejaculation behaviour — the results were significantly in favour of the control groups. For latencies to either mount, perform intromission or to ejaculate, the results were either similar or in favour of the untreated group.
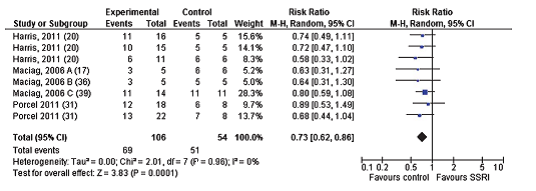
In conclusion, exposure to SSRIs showed substantial and lasting effects on sexual behaviour in rodents and these effects were not reversible after a drug-free period.
Impaired reproduction after exposure to ADHD drugs
We investigated whether mammals treated with ADHD drugs suffer from impaired fertility. For seven studies with clonidine, a drug also approved for migraines and elevated blood pressure, the rats that actually showed an interest in mating were not capable of impregnating the females due to no ejaculation or sterile sperm.
For nine studies with methylphenidate, the most used drug for treating ADHD symptoms, we found that female rats had delayed vaginal patency and the number of estrous cycles was halved. Fertility was also impaired in treated male mice, counted as number of embryos in untreated female mice. There were no differences when measuring number of stillborns or offspring or exterior malformations. However, interior malformations of the skeleton were seen. Generally the differences between groups diminished when the treatment started later in life or when a drug-free period was applied after treatment but before assessment of outcomes.
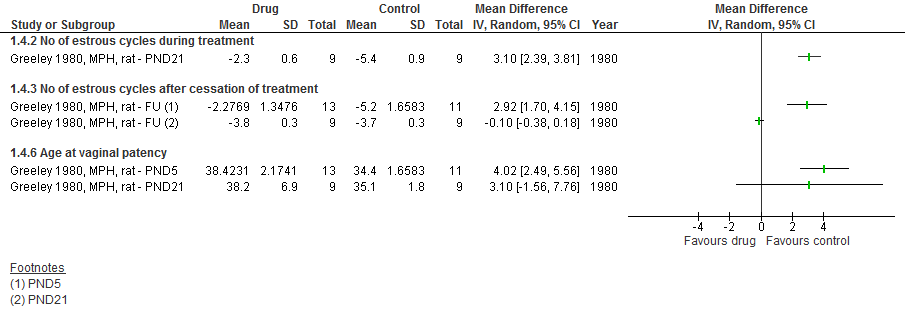
For one study with amphetamine there were no differences between groups for number of ejaculations and ejaculation latency.
In conclusion, exposure to ADHD drugs may impair fertility in rodents, but the impairment is somewhat reversible after a drug-free period.
Risk of bias in animal research based on these three systematic reviews
The heterogeneity of the studies was high; there were many differences between studies when choosing animals and strains, housing conditions, choice of drug, treatment length and route of administration of the drug, and there were highly varying test conditions even when measuring the same type of behaviour, e.g. anxiety. Number of animals per group was often low, which does not ensure sufficient power to conclude from the statistical analyses.
The quality of the studies was generally poor or poorly reported:
Selective reporting of outcomes mentioned in the methods section was present, but to a smaller extent. Often, nonsignificant outcomes data were not shared. Declarations of conflicts of interest and information about funding were lacking. Few studies reported on drop-outs, deaths or adverse events.
The majority of studies were described as placebo-controlled. A placebo-controlled study usually implies blinding of the observers, but this information was only given in detail for a few studies. No studies mentioned whether the caregivers and other personnel involved with the study were blinded. In animal studies, the caregiver and the observer are sometimes the same person, which means that the use of a placebo has not necessarily led to blinding of either of them, since there could be differences in, for example, the colour of the food, animal behaviour or animal size.
In animal studies it is important to randomize in three different ways (link). First, you have to randomize the animals to either the intervention or the control group to ensure that you do not introduce a bias, e.g. from litter effects.
Secondly, you should apply randomized housing. For example, rats and other rodents are very sensible to light and temperature. If all the intervention rats are placed in the top boxes of the racks there will be a temperature and light difference compared to the control animals in the boxes at the bottom. And these differences introduce a bias in the study, as they cause altered behaviour or altered hormonal regulation.
Lastly, to further minimize bias the researchers should apply random outcome assessment, because animals (just like humans) experience diurnal variation. Therefore, if assessing the intervention animals first (before lunch) and the control animals later, a systematic error will be introduced. For all three systematic reviews, no studies described having used all three ways of randomization and very few described having used two or even just one.
The neglect of considering bias reduction generally causes overestimates of effect and underestimates of harms (link). Therefore it is of utmost importance for future animal research that they are better designed.
Another bias to consider is publication bias. Two of our mentioned reviews have been published, but the third one has not been accepted for publication despite submission to several journals.
Within the last two years, we submitted the paper to four different journals that accept systematic reviews and animal research (Journal of Risk and Safety in Medicine, PLoS Biology, PLoS One, Psychopharmacology) and resubmitted it to PLoS One. The reasons given for declining the paper were that it was out of scope, did not add anything new, too few studies, the quality of the studies was too poor, and we did not divide the studies according to the mechanism of action of the drugs.
It is not our fault that the animal studies were of poor quality and it definitely has merit to publish our review, as it could help in the planning of better future studies of potentially great value to humans.
Discussion
Despite the studies’ shortcomings, we found that there are important findings in our work: psychiatric drugs do cause long-term impairment and harms.
The ARRIVE guidelines were introduced in 2010 to improve the poor quality of reporting for animal studies, but we found that even after 2010, the studies continued to be poorly designed and published.
We cannot dismiss the available preclinical studies in psychiatry entirely, despite poor quality in methodology and reporting. To advocate for better studies, systematic reviews are needed. By increasing focus on the risks of systematic errors and on selective reporting, animal research in psychiatry could improve in accuracy and transparency.
Since animal research is the foundation for moving on to clinical trials despite its poor quality, it is likely that this leads to many superfluous trials in humans based on false hopes and thus causes an unjust approval of trials. This of course happens at a huge economic expense for the funder, but also leaves a risk of serious and non-serious adverse events for the participants in the trials and subsequently the patients. If the harms of psychiatric drugs were better known from preclinical studies, less money and resources might be wasted on the development of new drugs.
If a researcher encounters the ARRIVE guidelines for the first time during the submission process, it is obviously too late to correct for systematic errors introduced during the laboratory work. Therefore, we believe that research ethics committees should ensure that protocols live up the ARRIVE guidelines.
Regulatory agencies are also relevant stakeholders, as they work to ensure high quality of the treatments available to the citizens. Regulatory agency employees should be educated to incorporate the ARRIVE guidelines into their daily work with the scientific literature. We believe that with increased rigour in animal research, the number of false leads will decrease.
In order to improve on animal research and its translational value we advise the following actions be taken:
• We need to assess why the studies are methodologically weak and poorly reported and why this is accepted by the field in the first place.
• All parties involved with animal research, researchers, funding bodies, peer reviewers, journal editors, and also research ethics committees and regulatory agencies, should take action to ensure that it becomes mandatory to adhere to the ARRIVE guidelines. For changes to happen, a special educational program should be set up in each country that educates students, researchers, research ethics committees and regulatory agencies.
• We should set up a trial register for animal studies — like for human trials — to improve all areas of animal research. The trial register should hold information about ethics approval processes and protocol information, thus creating more open and transparent research.
Conclusion
We have shown that harms from exposure to psychiatric drugs do exist, also on a long-term basis. We know too little about long-term harms, and our research reported here is not exhaustive. We have the option of gaining high quality knowledge from animal studies to improve the foundation for moving on to human trials, but these animal studies are still lacking. Animal psychiatric research is being poorly conducted or poorly reported. We urgently need to take action to ensure improved quality of animal psychiatric research and to educate all stakeholders in animal psychiatric research for this research to be a useful source of evidence.















Before psychiatric drugs-medication I was a reader of books. I have a fair sized collection of books from that time. Today I don’t read ( I find the world too noisy) and I wonder if the drugs affected my brain.
A reading test is one that no animal study can duplicate.
Report comment
I am sorry to hear that. And you are right, we cannot do a reading test in animals. But we can analyse the test that research whether drugs can improve reading difficulties with a critical mindset and always with an analysis of biases: study design, selective reporting, and so on.
What the sparse literature on harms in animals shows is that there are reason to be careful with these drugs. And maybe a reading test is not necessary when we see that the behaviour of the animals is affected. Maybe that should be enough to make us stop and think it over again. I hope it will.
I still fail to understand why drugs suddenly (?) is the solution to everything. Who set those norms in the first place?
Report comment
I thought animal research was now banned. Is PETA still active and if they knew of your studies they would be outraged.
Abuse is abuse whether with human and or animal subjects exposed to possible toxic chemicals.
I would suggest you read about the LSD and other experiments foisted on willing and or unaware subjects. Also read Richard Adams “Plague Dogs.”
Once I and others felt clinical trials were at the cutting edge of the best medical thinking of the time. I have learned with rue and regret the powers that be co-opted much of medical research for the advancement of fame, wealth , and glory.
There is a wide spectrum on what is and is not acceptable in research.
There needs to be a firm ethical foundation and do no harm part and parcel of the process.
Dialogue and openness and knowledge of history needs to be involved.
Edward Jenner’s inoculation of himself and family members- worthwhile or not?
Samelweiss and his concern about hand washing .
Lister and his concern about clothing of medical folks.
Madame Curie and her husband and their deaths.
Terry Tempest Williams and her accounts of families during the era of atomic testing and their resultant medical problems.
Is this the best we can do?
There has to be a better way.
Report comment
To my knowledge animal research is not at all banned. It seems that grants for preclinical research is on the rise. Do you have more information on your statement, I would love to learn more about the state of animal research.
I agree with you on the ethical part of research – it seems to have vanished. All is about money and not the patient.
And I ask as you do – is this the best we can do? Do we really need to be blinded like that? How come no one thinks for themselves any more? Where did the critical sense go? When did we just accepts the statement that drugs fix everything. It is scary.
Report comment
Pia, This is in some ways a large can of worms but I think the time has come to talk and begin discussions. I have knowledge coming from multiple side of the issue from both a personal and professional stand point.
US Primate Centers – PETA heavily protested these centers and found issues. At the same time, these centers worked on cutting edge AIDS research during the awful epidemic. Some primate centers have now been closed.
Most medical researchers have used animals because they thought it was better than say the use of research on unknowing or uninformed human subjects. Look up Tuskegee Institute history of horror.
The Nazis used concentration camp children for medical research look up the accounts of the survivors of the twin research.
Read “Refuge” by Terry Tempest Williams and her account of the effects of nuclear testing on her extended family members.
Check out the movie “Lorenzo’s Oil” a film version of a true account on how a father helped find a solution to his son’s genetic disease.
The urge to find help, to find a cure, to find the proverbial bullet runs deep for family members.
The urge to find better ways of fighting protecting the country runs deep in governments.
The urge to find more profit, more money runs deeps in for profit and even nonfood profit systems and research is then used upside down.
The focus is on how to keep people addicted and profit flow not only flowing but more and more.
With chemicals used by agriculture the research is on how to increase profits no matter what the chemicals are doing to the biomes and ecosystems.
Read Rachel Carson’s “Silent Spring”
Even a so called simple product like infant formula can create issues because it is given to developing countries therefore allowing innocent infants a portal for cholera and other diseases where water is not abled to be boiled.
At one time medical researchers were transplanting heads of animals on to dogs. True. Names withheld.
I also am aware of medical researchers who are good decent folks and are aware of the need of families and patients who are facing life long medical problems and pain. They can be forced into silence when they become aware of ethical issues. NDL’s and gag orders and threat of professional obliteration. See some of the Hollywood movies that centered on doctors caught in research nightmares.
For decades state institutions and schools for the disabled were the perfect place for research. Silence and lack of power perfect place to work.
Abuse and those who participate in it are bullies and act like typical offenders.
Whenever their is lack of truth, the push for profit over ethics, bullying of whistleblowers or potential whistleblowers, whenever there is the smell of stink coming from CEO’s CFO’s lavish lifestyles and perks given to professionals to keep them quiet or aid and abet the goal of the corporation, institute, or system ` unethical thinking, and justifying is looming and looms large.
In the eighties the hospital I worked for pushed research on all departments. It was voluntary but not voluntary. We did research projects that we had no heart or interest in and the results were compromised but put in because of the pressure.
Small beans, but that aura of culture kept growing and growing.
Check out how rabbits were killed for pregnancy tests. Check out research done by cosmetic industries.
Some folks would say PETA over steps but I think they need to be listened to and respected for their viewpoint just as families members and patients need for help need to be listened to and respected.
Ethics needs not to be sidelined as a pain but as a full partner at the table of medical research.
Think tanks funded by neutral funding resources with all concerned folks dialoguing.
Doctors need to hear parents, researchers need to hear and see patients face to face, funders need to talk to all involved.
Profit should be taken off of the table of consideration.
Good things have happened with medicine and to her sciences but just because good things have happened or are happening in no one removes the ethical onus for all involved to use ethics as a guiding light so that shadows can disappear or kept to a bare minimum until animals are never used.
The use of cadavers, dead embryos, all need a long and deep conversation.
We not fear dialogue and strong feelings and tiring thinking. We just need to do it and move on to a better way of acting and creating helpful information.
As you can tell, the arts also need to be pulled in. There are great television episodes, novels, movies, plays, folktales that can provide perspective and guidance.
An Ethics Profession using all of this could help.
There are some and those who work or work in hospitals know about Tumor Boards and meetings trying to figure out what happened to a medical nightmares. The Ethical Review Boards that really are more show than anything else because they are considered dangerous and or superfluous.
We are all humans, imperfect beings at best.
We all need to listen to our better angels in every way. There will aways be issues, the having to take the better choice versus the best choice.
But as long as that choice was well considered and worried on that is okay.The concept of trying is the true golden rule. We cannot ask anymore. Good and true caring ethical human effort.
Report comment
Thanks. I more or less know what you present here. I personally work for a better world. However, the opposition is massive and strong, so it is hard work. I work anyway, everyday, the best I can.
Report comment
yes there is a better way, but it requires lots of patience and will power,
Report comment
Together, when we are enough people, we can summon enough will power to make a change.
Report comment
Please leave the poor animals alone. Why do we have to keep proving that poison is poison?
Report comment
Well said oldhead! Not only poisons – these researchers also use authentic sounding neuro-jargon to justify how these ‘medicines’ work (saying things like “blocks the reuptake of both serotonin and norepinephrine,” etc). All these give the impression to innocent patients that scientists have fully figured out how the brain works!
Report comment
I agree, with both of you. I did not perform animal studies. I did a systematic review and meta-analysis collecting the evidence from studies, that others performed. I id this only to show that animal studies are weak evidence as they were poorly performed. Thus the trials were sad to say a waste of money and animals. I want this to change one way or the other. However, besides this I also work to help people help themselves, so they will never enter the psychiatric system. In that way pills are not needed and then neither is the research. I personally hope for these drug companies to declare bankruptcy some day.
Report comment
How many SMI seriously mentally ill have children? No one talks of this.
That IS what animals do. Eat food , and reproduce.
With the label and psychiatric drugs the person can not find employment and does not have a reason to work without a family.
It IS a jungle out there and almost everyone is working for their own interests.
The most surprising is when your own family wants you drugged out the the fear of the unknown future/fear of mental illness. The King and Queen want to stay in power and will do anything to keep it.
Report comment
Sad but true. I hope for the pressure against psychiatry to increase so that eventually it will break down completely and rise again in a whole new format. I do my share of things to participate in maintaining or increasing the pressure.
Report comment
Many studies have shown that any positive effects that psychiatric drugs show are due to the placebo effect, and the ‘chemical imbalance theory’ is a myth. So, I do not understand why the conclusion of this article says “we need to ensure improved quality of animal psychiatric research.” As I see it: We do not need to research psychiatric drugs at all for mental issues – it is the WRONG approach.
Report comment
Oh, I completely agree that we do not need to research any more drugs. However, a lot of people disagree and still believe the chemical imbalance theory. And they dont listen to facts that goes against their belief, but they listen to different kinds of information. Still, to my knowledge, animal research is the foundation for doing clinical trials – and we therefore proceed to trials in humans on a biased foundation. Which is so wrong. So to open people’s eyes we need to make a constant and ever increasing pressure to make people see. At some point they cannot close their eyes any longer. But it takes time. And during that time animal research is still the foundation for doing clinical trials. So I work at various levels to make the dream come true that we dont research in drugs for mental illness anymore.
Report comment
Thank you for your reply Pia. Yes, I agree it is best to proceed slowly in ‘educating’ these researchers.
I thought of adding that – observing animal models maybe useful for learning the function of organs like the liver, pancreas, etc., because such investigations can directly lead to treatments. But scientists forget that when it comes to the brain/mind, things are different and that they make one fundamentally false assumption. This is: because brain activity is CORRELATED with thinking, they assume that the organ brain has to be somehow treated when someone has mental issues (this has also led to the assumption that the ‘mind’ is located inside the head!). They forget that it is the mind (consciousness) itself that studies the organ brain and the brain cannot talk for itself.
Additionally scientists ignore the fact that it is HUMAN EXPERIENCE that brings about changes in brain chemicals (and also result in structural changes in the brain – i.e., neuroplasticity and epigenetics). Mice subjected to various psychological stresses (e.g. being restrained) clearly show many changes in the brain [see for example the article https://www.ncbi.nlm.nih.gov/pubmed/22127301%5D – these changes are reversible through psychological means (e.g. when stressed, restrained animals are released as described in the same article). Even studies conducted with jugglers, taxi drivers, etc., have shown that it is psychological experience that brings about changes in the brain. Mindfulness practices are known to change the structure and function of the brain in positive ways – one recent study showed that different types of meditation change the brain in different ways (see: http://advances.sciencemag.org/content/3/10/e1700489 )
So, unless these researchers are encouraged to see things with a broader lens, wasting a lot of money to find medicines for the brain will continue.
Report comment
In our research we used studies with animals that were not primed for any certain trait or behaviour. We used non-modeled animals. We did not think that adding one drug or operation or genetic modification would give the best answer to the results of being exposed to a psychiatric drug. There we used healthy animals.
However, that being said, this research was made to try to make animal research responsible, if animal research has to be the step before human trials. Then do not waste resources and animal lives by doing flawed research that does not aid to anything anyway.
My own personal view is that we should not research these drugs anymore, not in animals and not in humans. They do not work, they cause tremendous harm and you cannot solve emotional distress with pills.
One journal declined our manuscript because we did not divide the drugs according to mechanism of action. I thought that was a weak response to the decline. I am not interested in the mechanism of action, I am interested in what we see, when we watch the animal, no matter what mechanism of action might be described.
These so-called mechanims of action are not to be trusted. Regulating one neurotransmitter affects the whole milieu, including all the other neurotransmitter in the are. It is the chemical imbalance theory being used as an argument again, despite the complete sillyness of it…
Report comment
Thank you Pia. I wish you the very best for your future endeavours!
Report comment
“…we don’t research drugs for mental illness anymore.”
Psychiatry would have to get its story (lies) straight.
Applying a physical treatment for a non-physical illness is crazy.
https://en.wikipedia.org/wiki/Biological_psychiatry#Criticism
Report comment
So true. But the world is rigid and hold firmly onto beliefs. Despite them being lies.
Report comment
instead try on humans or tribal or voluntary humans
Report comment
“Tribal”?
Report comment