The National Institute of Mental Health (NIMH) has violated a key U.S. transparency law aimed at curbing research waste and evidence distortion in medicine on numerous occasions, data compiled by STAT News shows. In total, NIMH failed to post the results of 11 of 24 trials covered by the law onto the publicly managed trial registry Clinicaltrials.gov within the twelve month deadline stipulated by the law. Three of those clinical trials remain without results on the registry to this day.
Congress passed the FDA Amendment Act in 2007 to ensure that the results of clinical trials are made public irrespective of whether their outcomes are positive or negative. It was hoped at the time that the new law would counter widespread reporting bias and evidence distortion in medical research, and restore some balance to a medical literature that has repeatedly been shown to systematically overstate the efficacy of drugs and underreport their harms. However, over ten years on, the law remains unenforced; not a single fine has been imposed.
Among those routinely violating the law are U.S. government institutions, among them the NIMH. Not a single major institution has a track record of full compliance. In total, out of 1,286 taxpayer-funded clinical trials, 923 violated the transparency law by posting results late or not at all. Out of those, 163 are still missing results on the registry.
Out of the thirteen largest public trial sponsors, the Department of Defense (56% of trials missing results), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (46% missing), and the Veterans Administration (34% missing) are by far the worst performers. NIMH occupies middle ground with 17% of trials missing results.
Clinical trials completed >12 months ago without summary results, in %
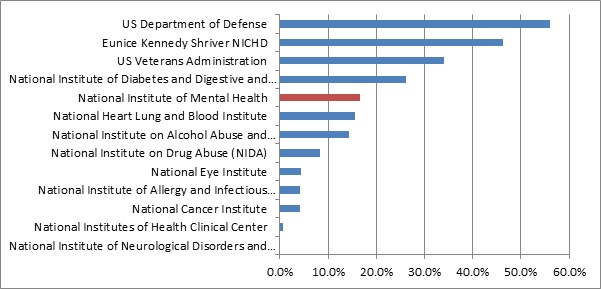
Compared to the top performers, NIMH’s track record looks distinctly unimpressive. NIMH completely dropped the ball on 4 out of only 24 applicable trials in its portfolio. In contrast, the National Institutes of Health Clinical Center managed a portfolio of 154 trials, and posted results for 153 out of those.
When challenged about their failure to post the summary results of clinical trials onto registries, institutions sometimes argue that the outcomes of many of those trials have been published in academic journals, making disclosure in registries unnecessary. However, research shows that summary results often provide a more robust and accurate picture of trial outcomes, including serious adverse events, than journal articles do. They thereby reduce the potential for reporting bias and evidence distortion, including a widespread form of research misconduct known as outcome switching.
For this reason, numerous groups — including the World Health Organization, Cochrane, and the global anti-corruption organization Transparency International — have called on governments to ensure that the summary results of all clinical trials are posted on registries with 12 months of trial completion, without exceptions. (The FDA Amendment Act only covers a small minority of all clinical trials.)
The failure of NIMH to post summary results for all of its trials is especially concerning because evidence distortion appears to be remarkably widespread in journal articles discussing trials of antidepressants and other psychiatric drugs. In addition, NIMH’s frequent failure to post summary results within the 12 month time limit set by law slows down the urgent quest for more effective treatments for mental health conditions. For example, NIMH has yet to post the results for trial NCT00794625, in which persistently aggressive children with ADHD were given Valproate and Risperidone to discover whether this would reduce their “defiant, belligerent, and otherwise aggressive behaviour.” The study was completed nearly five years ago.
Last year, over twenty major institutions worldwide jointly committed to posting the results of all their clinical trials within a 12 month time frame. U.S. government agencies are conspicuously absent from the list of signatories. An initiative that flags recently completed trials as soon as they fall foul of the law has detected four failures of the National Cancer Institute to post results on time within the past month alone.
With policymakers in Washington always on the lookout for opportunities — some may say excuses — to cut research budgets, NIMH and other taxpayer-funded medical research institutions may do well to recall the 2016 threat by then Vice President Joe Biden to cut off funding for researchers who fail to report clinical trial results in a timely fashion.
Campaigners for clinical trial transparency have long argued that patients deserve a better deal. If NIMH and other U.S. government institutions do not get their act together soon, Congress may start asking why it should fund research that fails to meet basic transparency requirements, and conclude that taxpayers too deserve a better deal.
Note: The author would like to thank STAT News for sharing its data set. The data set showed that as of September 11, 2017, NIMH has failed to post summary results for four clinical trials that were at that time overdue. In the meantime, NIMH has submitted the summary results for one of those four trials (NCT00183443). NIMH has still not posted results for the other three trials.














The dog has eaten my report! That’s why I gave it late.
Report comment
Sylvain, your comment is not quite complete…should read:
The dog has eaten my report! That’s why I gave it late…and why it is a lump of dog poop!
Report comment
There’s probably a good reason “NIMH has yet to post the results for trial NCT00794625, in which persistently aggressive children with ADHD were given Valproate and Risperidone to discover whether this would reduce their ‘defiant, belligerent, and otherwise aggressive behaviour.'”
Risperdone probably grew breasts in many of these little boys.
http://risperdalboys.com
Oops! Not a good idea, NIMH.
What ever happened to Thomas Insel’s promise to stop wasting taxpayers’ dollars for funding research into the “invalid” DSM disorders?
Report comment
NIMH payed Insel with money they received from taxpayers. Sickening.
No compassion, honesty, or conscience in any of these bureaucrats.
Like C.S.Lewis said in his intro to The Screwtape Letters: “I prefer bats to bureaucrats.” Bats are warm blooded at least.
Report comment
To spend all that time researching and then not provide the findings is ludicrous.
Report comment
They must sincerely feel that since the practice of Choreographed Kangaroo Court Long Term Commitment hearings are already in effect established , that it is their inalienable right , since they designed the clinical trials , to present whatever results they choose wherever and whenever they choose to or not at all. They must feel it is only a matter of time till they in effect can establish the practices and results , which they choose to establish . After all if we look around we can see what they’ve accomplished already.
Report comment
So the NIMH broke the law? Guess that means fines and/or jail time for the higher ups eh? Because if nothing happens it means the law means nothing. If the law applies it should apply to everyone equally. Not one set of laws for governments, corporations, and rich and wealthy elite where they can get away with mass murder while another set of laws for everyone else where they can’t get away with growing or smoking a plant or saying words because it might offend someone.
Nothing will change until people accept that THIS IS INTENTIONAL. Manipulating study results, downplaying negative effects while talking up any minor positive even if they have to manipulate a negative into a positive, posting positive results but not negative results, promoting the psychiatric agenda of chemical imbalances and broken brains and drug interventions as the best and first line interventions, pushing psychiatric drugs as ‘safe and effective’ when many have been proven to be little more effective than placebo yet include the risk of many adverse effects including homicidal and/or suicidal thoughts and/or actions, ignoring evidence that ‘chemical imbalances’ never existed (except the ones caused by psychiatric drugs) and ‘mental illnesses’ are not real illnesses but clusters of personality traits, behaviours, and emotions that are clustered together and given a label and voted into existence.
What these nutbars in power are doing is ON PURPOSE. They didn’t accidently forget to file their paperwork. They didn’t misspeak saying psychiatric and pharmaceutical drugs are “safe and effective”. It’s not a woopsie that they hire “Key Opinion Leaders” to influence others in their profession. They didn’t make a mistake hiring thousands of drug reps and lobbyists to influence policy makers and script writers. This is all intentional. Nothing will change while you people look at psychiatry and look at what they are doing like it’s a ‘mistake’ or an ‘accident’ or ‘misguided’ or ‘doing it wrong’. What they are doing is by design, they are doing exactly what they intend to do. And what they intend to do is maintain and expand the status quo of power, profit, and control by continually making up mental illnesses and pointing the blame at broken brains and individuals (while ignoring the environment, society, and government policy) and pushing for everyone on the planet to be monitored 24/7 and drugged up to their eyeballs from cradle till grave and those who question or step out of line will get a more ‘dangerous’ or ‘crazy’ label (or given a low social credit score and banned from most services) and given more powerful drugs or electroshocked into a dribbling shell of a human who can no longer think for themselves or question the increasingly insanity of those in power.
Report comment