A new study tested transcranial magnetic stimulation (TMS) for treating people with a bipolar disorder diagnosis. The researchers found that actual TMS was no better than a sham (fake) treatment.
The study was led by Lakshmi N. Yatham at the University of British Columbia Hospital in Canada and published in JAMA Network Open.
The specific type of intervention used is intermittent theta-burst stimulation (iTBS), a type of TMS. The researchers targeted the left dorsolateral prefrontal cortex (LDPC) in the brain. The goal was to relieve depression symptoms in people diagnosed with bipolar disorder.
iTBS was used, and the LDPC was targeted because these are currently popular TMS methods in depression treatment. Yatham writes:
“There was no evidence of antidepressant superiority for active iTBS over sham iTBS, and safety is uncertain because 1 hypomanic switch occurred with active iTBS and a second occurred during the open-label phase.”
That is, people randomly assigned to the fake treatment did just as well as people who received actual iTBS. Additionally, two people undergoing actual iTBS became hypomanic—compared with none who were receiving the sham treatment.
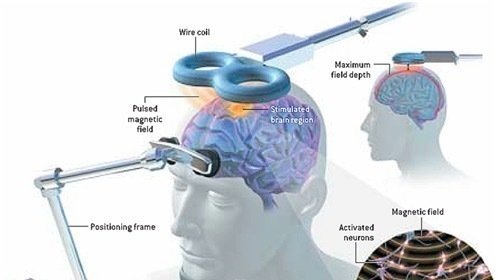 The study was double-blinded, meaning that neither the participants nor the researchers knew who was receiving iTBS and who was receiving the sham treatment. The randomized, controlled trial lasted for four weeks, with an additional four-week “open-label” phase during which there was no sham control group.
The study was double-blinded, meaning that neither the participants nor the researchers knew who was receiving iTBS and who was receiving the sham treatment. The randomized, controlled trial lasted for four weeks, with an additional four-week “open-label” phase during which there was no sham control group.
The 37 participants were required to have a diagnosis of bipolar disorder I or II, be currently experiencing a major depressive episode, and be currently taking a mood stabilizer and/or an antipsychotic drug. They were also required to have had no “clinical response” to the drug—that is, for the participants in this study, mood stabilizers and antipsychotics did not work to improve their symptoms.
People who had previously had no response to TMS were excluded, as were people who were suicidal, experiencing psychosis or substance abuse, or undergoing psychotherapy.
The study was intended to include 50 people in each group (totaling 100). However, the study was canceled early because TMS was so inadequate. Response rates were very low: 3 of the 19 people in the sham treatment group improved, while 3 of the 18 people in the active treatment group improved.
After eight participants dropped out, 29 participants completed the four-week randomized trial. 21 people then agreed to enter the open-label phase. Five of them dropped out, leaving 16 people to complete the open-label trial. Four of the 16 remaining people “achieved clinical remission” during the open-label phase—but there was no control group to compare these results to, so they could also have been due to the placebo effect or regression to the mean.
Although the researchers write that “blinding integrity was preserved,” they note that about half of the people in the study correctly guessed whether they received the active treatment or not. Additionally, people were far more likely to improve if they believed they had received the active treatment—which is evidence of a strong placebo effect.
Surprisingly, TMS is approved by the FDA for the treatment of depression for people with any diagnosis, including bipolar disorder. The researchers note that this is based on extrapolating findings from studies on people with a depression diagnosis rather than any actual evidence on bipolar disorder.
Nonetheless, this isn’t the first study to demonstrate the ineffectiveness of TMS. A 2016 sham-controlled study of a different type of TMS (sequential bilateral repetitive transcranial magnetic stimulation) similarly found that the active treatment was ineffective in those with a bipolar disorder diagnosis.
Even in the treatment of people with a depression diagnosis rather than bipolar disorder, the evidence for the effectiveness of TMS is unclear. Three large, multisite trials found that TMS was ineffective.
A review by the Clinical TMS Society frames these findings positively, but the results are clear:
- The 2007 Neuronetics trial: Improvement after four weeks was not significantly different between active and sham TMS groups (p = 0.06). A p-value of less than 0.05 is usually considered statistically significant (sometimes, a p of less than 0.01 is used for an even stricter definition). The conclusion that this was “safe and effective” was regarded as having “the potential to mislead readers” by other researchers.
- In the 2010 OPT-TMS NIMH trial, 15% of people in the active TMS group improved (versus 4% in the placebo group). Although this difference was statistically significant, 85% of the participants did not improve after receiving TMS.
- The 2015 Brainsway trial: Although initial results were promising, remission rates for “Deep TMS” at the final outcome point of 16 weeks were not significantly different between active and sham groups (p = 0.15).
****
McGirr, A., Vila-Rodriguez, F., Cole, J., Torres, I. J., Arumugham, S. S., Keramatian, K., . . . & Yatham, L. N. (2021). Efficacy of active vs. sham intermittent theta-burst transcranial magnetic stimulation for patients with bipolar depression: A randomized clinical trial. JAMA Netw Open, 4(3), e210963. doi:10.1001/jamanetworkopen.2021.0963 (Link)















I’m sorry but I think we should not present this as “ineffective”, but as downright dangerous and a most idiotic and selfish moneymaker that is profiting from harming people.
We are at a place in science where we cannot even diagnose nor treat nor operate on most back/ear and the majority of physical issues and disabilities.
Talking about brain treatments, the brain being the least understood, (actually not understood at all,) as ineffective, makes it sound as if the practice itself is okay, that the application is okay, just ineffective.
It is downright crazy for these things to make it into public arenas. They are dangerous.
Report comment
Did you catch how they required people who had no clinical response to deadly neuroleptic and other drugs to keep taking them? “Hey, those deadly drugs didn’t help you, so let’s have you keep taking them and now we will zap your brain.”
Report comment
“No result” is apparently no reason not to continue with “treatment” in the world of psychiatry. Continuing “treatment” is all that matters. If it doesn’t work, we double the dose. If that doesn’t work, we add another drug. We continue until the person either lapses into apathy or dies. That’s psychiatry in a nutshell.
Report comment
And what about the 8 dropouts Sam? After all my experience with folks who have been harmed by TMS, a lot of them have a very severe reaction after the first treatment. I would assume those folks had some sort of injury. Some of them don’t recover from just the harm of a single treatment as well.
It is really a serious injustice that dropouts are not evaluated or considered for injury resulting from the treatment. It really is a massive cheat.
Report comment
Will America ever stop wasting American taxpayers’ money, by ending the funding into the already debunked as “invalid” DSM disorders?
https://www.nimh.nih.gov/about/directors/thomas-insel/blog/2013/transforming-diagnosis.shtml
Report comment
Thank you so much for covering this Peter.
This is really a statistical reflection of the fraud and great harm TMS is. Without this type of analysis, unfortunately some people may never really believe that it is useless and a harmful mutilation of our brains.
Report comment
I had TMS for unipolar depression. I was well aware of its questionable effectiveness. Unfortunately, like many things in life and in the mental health system the “best treatment” is limited to what you can get. I wish I could have accessed the effective treatments featured on Madinamerica.
Report comment
I started TMS for other reasons, Fibromylegia, stress, severe body pain, spine degeneration, Osteasthritis and nerve issues. However, working for FDA, especially in device regulatory agency, I was surprised, why I never heard of this device, as I am familiar with most of the devices. I wanted to find positive experience but finding all negative response. So, I read all blogs and now having a second thought. So far, I finished seven sessions and get severe headaches, which I never experienced before. I am not sure if I would continue the treatment at this point. I would like to educate about FDA So, this is what I am addressing 1) This TMS device Neurostar is not “FDA approved”. It is ‘FDA Cleared”. The difference is, FDA approved device require clinical studies and clinical data of the device usage from real patients. FDA Cleared is simply, a device, which is equivalent to the similar device in market, before 1978. For example, if there was a massager for head, which can simply use for the similar function and it was used in 1900, the manufacture can tell that TMS is similar to that device, and FDA can give the clearance. No data are reviewed or required by FDA. 2) FDA inspects device manufacturer but if the device is not very critical, it is not a high priority. Also, each investigator varies and one investigator might not find any issues, next investigator after two years may find issues. So, it depends on how deep the investigation was performed. Therefore, there was nothing I found on TMS. 3) The manufacturer’s promotion of “FDA Approved” is in violation of FDA Act. Some one should complain to FDA about this. It is a misbranded device. 4) FDA require doctors, user, patients and manufacturer to report any “Adverse events” to FDA (Link is http://WWW.FDA.GOV and search Adverse event reporting under CDRH). Anyone can report device malfunction, serious injuries and death to FDA. When an investigator inspects mfr., they review these complaints. However, if manufacture don’t report to FDA, FDA will not know about it. So, I highly recommend all bloggers to report events to FDA. This applies for food, drugs, devices etc.
Do not think, FDA is your police or protector. You are in charge of your own health, just like don’t think, doctors are your protector or they have magic wand. They are just like you and I , who can also have similar health issues. Just a simple example, if in your house, there are two people who ate same food but one may get sick and the other don’t get sick. Each person reacts differently to the drugs as well. I tried so many antidepressants, pain medicines but nothing helped. Even if you say, drugs are approved by FDA, so it works. You are wrong. I know how drug companies and device companies lobby and have laws in their favor. Same way, do you think, one drug will act to your body the same way, as it was to the other? I am frustrated because there are so many loopholes in our system. Doctors are brainwashed by pharma companies, favored by pharma companies and many times, they are hired by pharma companies to be either on their board, as a consultants, to do clinical research for them etc. The ethics requirement is to pay very nominal amount to the doctors and that is one of the FDA requirement for the doctors who participates in clinical trials. However, there are many other ways, doctors are paid to favor the pharma companies. as I mentioned before. Also, if there is a death or serious injury reported to FDA by the user or other doctor, most of the times, manufacturer blames doctor that he/she did not use the device properly as described, how to use. Many times, they employ or hire doctor as their consultant, who writes investigation report as either it is patient’s condition responsible for the injury, or the patient’s anatomy responsible for the injury and not the device. I have seen enough during my entire career with FDA. Even working for FDA, I myself experienced many adverse events in my family, including the death of a child due to the faulty device and I was frustrated that FDA did not take any action. I have seen legitimate lawsuit by patients but at the end, companies know how to get around proving the patient to be blamed for.
Report comment