Tag: conflict of interest
Malcharist: Fact or Fiction? Big Pharma, Psychiatry’s Key Opinion Leaders and...
Malcharist, by Paul John Scott, is a fictional account of one of psychiatry’s most influential key opinion leaders (KOLs), his ghostwriter, and a journalist on the trail of a big scandal in the world of Big Pharma.
Treatment Guidelines Should Not Be Written by Professional Societies and Insiders
John Ioannidis, a leading expert on research methods, takes a critical look at the way professional societies write treatment guidelines.
Antidepressant Adviser to UK Gov’t Quits After Conflict of Interest Row
From The BMJ: " Baldwin was the Royal College of Psychiatrists’ representative on , which was set up to review the potential effects of...
Conflicts of Interest Questioned in Royal College of Psychiatry’s Participation in...
From: James Moore, antidepressant withdrawal sufferer, on behalf of the 30 other signatories to today’s letter.
London, UK – A fellow of the Royal College...
A Blow to STAT’s Credibility: Ghostwriting/PR Influence
From HealthNewsReview.org: STAT recently published an op-ed praising the role of drug company sales representatives. The physician listed as the author has now revealed that he did...
Time for Full Transparency on Pharmaceutical Money
From The Toronto Star: Ontario law should require pharmaceutical companies to not only disclose payments to physicians but also make public their contributions to all...
Panels That Developed Treatment Guidelines Had Industry Ties
From STAT: A recent analysis found that a large portion of depression treatment guidelines was developed by individuals with financial ties to the pharmaceutical industry.
"Of 172...
Two Thirds of Patients See Physicians Who Receive Payments From Pharma
Study finds more patients are visiting physicians who have ties to industry than previously thought.
Many Patient Advocacy Organizations Are Funded By Industry
New research investigates the financial conflicts of interest (FCOI) of patient advocacy organizations (PAOs) in the United States.
“A Frenzy Of Lobbying On 21st Century Cures”
Kaiser Health News and NPR report on the immense lobbying effort aimed at passing the "21st Century Cures" Act which would fast-track FDA approval...
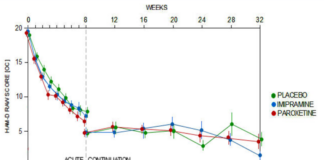
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Garbage In–Garbage Out: Systematic Reviews and Meta-Analyses can tell us a...
Well known Stanford University researcher John Ioannidis published a new paper this week criticizing the use and production of systematic reviews and meta-analyses, often...
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
“Deep Brain Stimulation: Unproven Treatment Promoted with a Conflict of Interest...
In the PLOS Mind the Brain Blog, James Coyne reacts to an article and editorial in JAMA Psychiatry reporting effects of brain stimulation therapy...
“Harvard Affiliates Protest at Tufts for ‘Pharma Fools Day’”
Students from Harvard Medical School joined students from Tufts University to protest Joseph DiMasi, who recently published a study that the students claim is...
Study 329: 50 Shades of Gray
Access to data is more important than access to information about conflicts of interest. It is only when there is access to the data that we can see if interests are conflicting and take that into account. Problems don’t get solved unless someone is motivated for some reason. We need the bias that pharmaceutical companies bring to bear in their defense of a product, along with the bias of those who might have been injured by a treatment. Both of these biases can distort the picture but it’s when people with differing points of view agree on what is right in front of their noses that we can begin to have some confidence about what we have.
Massive Number of Antidepressant Meta-Analyses Biased By Industry
A massive number of meta-analyses of antidepressant clinical trials have financial conflicts of interest and are unduly influenced by pharmaceutical companies, according to a review to be published in an upcoming issue of the Journal of Clinical Epidemiology. Researchers also found that meta-analyses with industry ties almost never report any negative findings in their abstracts.
Nominee to Lead FDA Removed Name From Recent Publications
Sheila Kaplan for the Boston Globe reports that Dr. Robert Califf, the Obama administration's nominee to lead the Food and Drug Administration (FDA), has removed his name from a series of scientific papers that he recently coauthored. The decision to remove his name, against publication ethics standards, has brought Califf under renewed criticism.
JAMA Editorial: “Confluence, Not Conflict of Interest”
Yesterday, the Journal of the American Medical Association (JAMA) released an editorial entitled “Confluence, Not Conflict of Interest: Name Change Necessary.” The authors argue that the phrase “conflict of interest is pejorative,” and a better term “would be confluence of interest, implying an alingnment of primary and secondary interests.”
Nominee for FDA Commissioner Has Strong Industry Ties
On Tuesday, the Obama administration nominated Dr. Robert Califf to be the new commissioner of the Food and Drug Administration (FDA). In a statement, director of the Public Citizen’s Health Research Group, Dr. Michael Carome called on the Senate to reject the nomination. He contends that Califf “racked up a long history of extensive financial ties to multiple drug and medical device companies, including Amgen, AstraZeneca, Eli Lilly, Johnson & Johnson, Merck Sharp & Dohme and Sanofi-Aventis, to name a few.”
Watchdogs or Show Dogs?
Beginning in the 1990s, a number of pharmaceutical and biotechnology companies began to set up bioethics advisory boards, ostensibly to obtain guidance about controversial ethical issues. Over the years, the ties between industry and bioethics have gradually grown closer, with companies setting up endowed chairs and hiring bioethics consultants. Yet very little is known about how bioethics advisory boards work. What exactly is their purpose? Do they prevent ethical wrongdoing, or do they provide ethical cover?