Tag: paroxetine
Researchers Find Paroxetine Harms Developing Brain
Researchers at Johns Hopkins test paroxetine on developing brain cells and discover numerous neurotoxic effects.
Antidepressant Withdrawal: An Unknown Disorder?
Antidepressant withdrawal is no longer an unknown disorder since knowledge on this topic has grown enough to be translated into practice. As proposed by George Engel in 1977, medical doctors, including psychiatrists, can observe and listen to their patients and develop a program to treat withdrawal and restore health.
Peter Groot and Akansha Vaswani: Tapering Strips and Shared Decision-Making
Doctoral candidate Akansha Vaswani interviews researcher and geneticist Dr. Peter Groot, who has led the development of Tapering Strips, a novel and practical method by which people taking certain prescription medications can gradually reduce their dosage.
Peter Gordon: Addressing the Divide Between the Arts and Medical Sciences
An interview with Dr Peter Gordon who describes himself as a gardener with an interest in medicine. He trained in both medicine and architecture before specialising in psychiatry. In addition, he is an activist and campaigner and has a range of creative interests including filmmaking, photography, writing and poetry.
Lawsuit Over a Suicide Points to a Risk of Antidepressants
From The New York Times: The recent trial of Wendy Dolin, whose husband died of suicide after starting the antidepressant paroxetine, demonstrates our need for more...
Change in Chicago: Playing Go
The jury was out for days. And when they came back it became clear they were wrestling with the issue of who to blame. This was like playing Go, where it can look like the black counters on the board have white encircled until white puts down one more piece and all of a sudden it wins.
Change in Chicago: The Dolin Verdict
Finally, faced with the twenty known and two possible suicides on Paxil during clinical trials, Dr. Kraus reluctantly conceded that 80% of the victims were over thirty. Whatever they had told the FDA, the risks of Paxil could not be confined to adolescents — and GSK knew it.
Wendy Dolin Takes on GlaxoSmithKline And Wins — For Now at...
In July of 2010, Stewart Dolin, a partner at the mega law firm Reed Smith, jumped in front of a subway train in Chicago, apparently suffering from akathisia caused by paroxetine. His widow sued, and the jury found GSK negligent in not informing doctors of the suicide risk
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
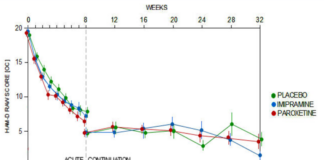
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Major Review Finds Antidepressants Ineffective, Potentially Harmful for Children and Teens
In a large review study published this week in The Lancet, researchers assessed the effectiveness and potential harms of fourteen different antidepressants for their use in children and adolescents. The negative results, familiar to MIA readers, are now making major headlines.
Who Will Guard the Guardians of Psychiatry?
The assertion that the so-called antidepressants are being over-prescribed implies that there is a correct and appropriate level of prescribing and that depression is a chronic illness (just like diabetes). It has been an integral part of psychiatry's message that although depression might have been triggered by an external event, it is essentially an illness residing within the person's neurochemistry. The issue is not whether people should or shouldn't take pills. The issue is psychiatry pushing these dangerous serotonin-disruptive chemicals on people, under the pretense that they have an illness.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
The Psychiatry Sandcastle Continues to Crumble
Psychiatry would long since have gone the way of phrenology and mesmerism but for the financial support it receives from the pharmaceutical industry. But the truth has a way of trickling out. Here are five recent stories that buck the psychiatry-friendly stance that has characterized the mainstream media for at least the past 50 years.
My Response to the FDA’s ECT Rule Change
I lived through forced ECT from 2005-2006 at the Institute of Living in Hartford, Connecticut. My experience with ECT was the impetus for me to become involved in the antipsychiatry and Mad Pride movements, although I am not entirely opposed to voluntary mental health treatment. The following is the comment I submitted to the FDA on its proposal to down-classify the ECT shock device.
“GSK Fined Over ‘Pay-for-Delay’ Drug Deals”
Reuters reports that GlaxoSmithKline has been fined $54.4 million by the UK for “market abuse in striking deals to delay the launch of cheap...
After the Black-Box: Majority of Children Starting SSRIs Still Receiving Too...
In 2004, the FDA added a black-box warning to SSRI antidepressants on the increased risk of suicide among children taking these drugs. A new study suggests that this warning has increased the proportion of children who begin an antidepressant on a low dose, but the majority are still receiving higher than recommended doses.
Study 329: Big Risk
Study 329 seems to fit the classic picture: It has Big Pharma ghostwriting articles, hiding data, corrupting the scientific process and leaving a trail of death, disability and grieving relatives in its wake. But is it at fault alone? Both Big Pharma and Big Risk (the insurance industry) were once our allies in keeping our hopes alive – in keeping our children alive and well. They are now a threat. And of the two – Big Risk is the bigger threat.
GlaxoSmithKline Accused of Hiding Paroxetine Results
The UK Times reports that pharmaceutical companies are actively lobbying to limit the release of clinical trial data to the public. Rather than limiting results and data to medical journals, new transparency initiatives are pushing for making the information publically available. The push for transparency comes in the wake of the reanalysis of the Study 329 data on paroxetine (marketed as Seroxat and Paxil), which found that the industry study had misconstrued its results.
Study 329: 50 Shades of Gray
Access to data is more important than access to information about conflicts of interest. It is only when there is access to the data that we can see if interests are conflicting and take that into account. Problems don’t get solved unless someone is motivated for some reason. We need the bias that pharmaceutical companies bring to bear in their defense of a product, along with the bias of those who might have been injured by a treatment. Both of these biases can distort the picture but it’s when people with differing points of view agree on what is right in front of their noses that we can begin to have some confidence about what we have.
Study 329: Conflicts of Interest
The BMJ states that it takes on average eight weeks from submission of an article to publication. The review process for Restoring Study 329 took a year, with a three-month review process involving six reviewers to begin with, and then a further four reviews in a four-month process, leading to a provisional acceptance in March that was withdrawn.
Study 329 in Japan
By 2002 GlaxoSmithKline had done 3 studies in children who were depressed and described all three to FDA as negative. As an old post on Bob Fiddaman’s blog reproduced here outlines, several years later they undertook another study in children in Japan. (Editor's note: This is a re-print, by David Healy, of a post by Bob Fiddaman)
SSRI Antidepressants Increase Surgery Risks
There is accumulating evidence that taking SSRI antidepressants increases the risk of bleeding and other complications during surgery, according to a review published in the British Journal of Anaesthesia.
Study 329: By the Standards of the Time
The controversy over “Study 329” on the effects of Paxil in teen depression has raised questions about the state of ALL medical research. I decided to look at the research for the most recent psychiatric drug approved by the FDA, a new antipsychotic called cariprazine or Vraylar. I located twenty studies of Vraylar on www.ClinicalTrials.gov, the U.S. government-sponsored registry for clinical trials. Three were still in process, and seventeen were completed. Not one had shared its results on the government website, a supposedly mandatory step.
Study 329: MK, HK, SK and GSK
It is appropriate to hold a company or doctors who may be aiming to make money out of vulnerable people to a high standard when it comes to efficacy, but for those interested to advance the treatment of patients with any medical condition it is not appropriate to deny the likely existence of harms on the basis of a failure to reach a significance threshold that the very process of conducting an RCT will mean cannot be met, as investigators' attention is systematically diverted elsewhere.