Tag: conflicts of interest
Psychotherapy and Psychosomatics: End of an Era for Independent Journals? An...
Giovanni Fava joins us to discuss the uncertain future of the journal 'Psychotherapy and Psychosomatics' which he edited for thirty years and which has been essential to our understanding of the impact of psychiatric treatments.
Antidepressant Trials “Hijacked for Marketing Purposes,” Researchers Say
About half of the large antidepressant trials are biased enough to be considered “seeding trials,” according to the researchers.
“Beware, Scientology Related”: How ADHD Experts Silence Criticism
We do not belong to the scientology movement, but this false accusation triggered an email correspondence that exposed the problematic happenings usually behind closed academic curtains.
False Positives in Brain Imaging, Unpublished and Missing Trials, and Conflicts...
In our Science News podcast, Peter Simons reports on false positives in brain imaging, unpublished and missing trials, conflicts of interest and more.
Screening for Bipolar: Have You Ever Been “Unusually Happy” for More...
A new questionnaire funded by AbbVie conflates antidepressant side effects with bipolar disorder and doesn’t actually meet the criteria for being considered “screening.”
Conflicts of Interest Questioned in Review of Prescribed Drug Dependence
An interview with Professor Sami Timimi, Psychiatrist Peter Gordon and campaigner Stevie Lewis, who talk about the potential for conflicts of interest with the UK Royal College of Psychiatrists participation in a Government-led review of Prescribed Drug Dependence.
Scientific Opinions — For Sale
From Medium: "This is where evidence based medicine is supposed to shine, by demanding randomized trials to prove that certain procedures/drugs either work or don’t....
Journalists Should Report Their Sources’ Conflicts of Interest
From HealthNewsReview.org: While researchers are usually required to disclose their conflicts of interest in medical journals, media outlets do not often require journalists to disclose...
Disclosing Corporate Funding is Not Nearly Enough
From The Chronicle of Higher Education: A number of studies have shown the significant problems that arise from financial conflicts in research. Disclosure of researchers'...
Researchers Challenge Industry-Friendly Depression Guideline
Review of a new mixed depression guideline reveals financial bias of guideline developers and lack of evidence supporting recommendations for prescribing of antipsychotics.
Dr. Vinay Prasad Takes on Big Pharma and Big Medicine
In this piece for STAT, Meghana Keshavan profiles Dr. Vinay Prasad, a physician who has become an influential voice in the medical community through his...
The Moving Basis of Mental Health Diagnosis
In this opinion piece for The Chronicle Herald, Dr. A.J. discusses the subjective nature of psychiatric diagnosis and the DSM. Citing research by Paula Kaplan,...
Robert Whitaker Refutes Jeffrey Lieberman; But Is Psychiatry Reformable?
When the neuroleptics-are-necessary-to-treat-schizophrenia myth falls, psychiatry is finished. And that is why the Goff et al paper was produced: a desperate attempt to maintain its position by a profession that is truly on the ropes. For psychiatry this is a death-struggle.
Brain Stimulation Research Lacking in Reproducibility and Scientific Integrity
Questionable research practices and poor reproducibility in electrical brain stimulation (EBS) studies.
Trials Comparing Treatments for Depression Favor Pharmacotherapy when Statisticians Involved
A meta-analysis looks at the effects of researcher background on study findings for trials comparing pharmacotherapy and psychotherapy for depression.
“As Americans Increasingly Seethe at Big Pharma’s Money Racket, Govt Is...
Martha Rosenberg, for AlterNet: “Pharma may be becoming one of the public's most reviled sectors but the U.S. government is in the process of...
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
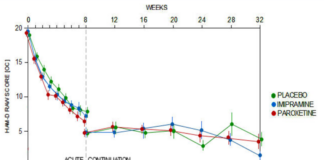
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
“Medical Groups Push to Water Down Requirements for Disclosing Industry Ties”
Pharmalot’s Ed Silverman reports on a Senate bill aimed at loosening requirements around the reporting of financial conflicts of interest between companies and physicians....
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
“Amid Public Feuds, A Venerated Medical Journal Finds Itself Under Attack”
The New England Journal of Medicine (NEJM) has come under intense scrutiny for delayed corrections and controversial editorials and articles. “The Journal and its...
“Doctors on Social Media Fail to Disclose Conflicts of Interest”
“A STAT examination of hundreds of social media accounts maintained by health-care professionals finds that while they often tweet medical advice, they almost never disclose potential conflicts of...
“The FDA Now Officially Belongs to Big Pharma”
“Robert Califf's ties to Big Pharma run deep and the Obama nominee for the FDA just sailed through the U.S. Senate.”
Article →
“Many in US Congress Hold Health Industry Investments”
“About 30 percent of senators and 20 percent of representatives held assets in biomedical and health-care companies, or in specialty funds set up to invest...
“Would Washington’s FDA Fix Cure the Patients or the Drug Industry?”
Legislation is being advanced that would speed up the FDA’s approval process for new drugs and medical devices, according to a report by the Pacific Standard. Pharmaceutical and medical device companies have been lobbying heavily to reduce regulations and are winning over bipartisan support by attaching these measures to increased mental health funding.