Tag: clinical trials
Pharma Data Sharing Efforts Off to a Slow Start
Researchers discuss the preliminary results of clinical trial data sharing efforts by pharmaceutical companies and other groups.
“Healthcare’s Dirty Little Secret: Results from many Clinical Trials are Unreliable”
For The Conversation, a team of researchers from the University of Aberdeen report that a substantial number of research trials produce unreliable results. “If that...
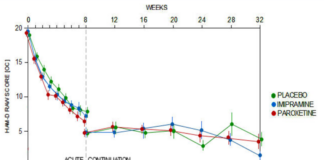
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
Clinical Trials Underreport Harms of Antidepressant Medications
A group of researchers recently found serious bias in the reporting of harm due to adverse events in antidepressant medication clinical trials. They report...
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
“New Federal Rules Target Woeful Public Reporting of Clinical Trial Results”
For STAT, Charles Piller covers new federal rules requiring stricter reporting for researchers conducting human studies. Previous investigations have documented widespread noncompliance with such...
“In Clinical trials, For-Profit Review Boards Are Taking Over for Hospitals....
Sheila Kaplan for STAT News: “The modern history of science is littered with studies in which participants were harmed because researchers failed to take...
“Jury Smacks J&J with $70M in Damages in Latest Risperdal Breast...
Fierce Pharma reports: "Johnson & Johnson ($JNJ) is fighting more than 1,500 legal claims that its antipsychotic Risperdal triggered breast development in boys, and...
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Lancet Editorial Points to “Trouble with Psychiatry Trials”
While clinical trials make up the “bedrock of evidence-based medicine” in other specialties, psychiatry faces a number of both ethical and scientific problems related to its use of randomized control trials. According to a new editorial in The Lancet Psychiatry, the field of psychiatry research has particular problems with ethical issues in recruitment, inaccurate classification systems, and controversial placebo comparisons, and then, once the studies are finished, it often remains unclear what the “outcomes actually mean for people’s lives.”
“BMJ Editor Fiona Godlee Takes on Corruption in Science”
In this video from CBC News, BMJ editor Fiona Godlee takes on “corruption of the scientific process.” "There will be commercial pressures, academic pressures,...
“The Outcome of My Clinical Trial Is a Mystery”
Writing for the Atlantic, Emma Yasinski confesses that she was enrolled in a clinical trial for an invasive experimental surgery at seven years old but, as the results were never published, she may never know how well it actually worked.
“6 Hospitalized, One of Them Brain-Dead, After Drug Trial in France”
Six men were hospitalized and one was pronounced brain dead after participating in a phase 1 clinical drug for a mood, anxiety, and motor dysfunction drug manufactured by Bial and administered by Biotrial. Carl Elliott, a bioethicist at the University of Minnesota, said investigators should look into questions like how much the men were paid and whether they properly consented to the trial. “Many Phase 1 trial volunteers are poor and unemployed, and they volunteer for trials like this because they are desperate for money,” he said. “This means they are easily exploited.”
“Martyrs to Science? When Research Participants Die”
Neuroskeptic covers a short article by Susan Lederer that appeared in the American Medical Association Journal of Ethics discussing what happens when research participants die.
$8 Million Awarded to Family Of Man Who Died in Risperdal...
A California jury ruled that Johnson & Johnson’s Janssen Pharmaceutical and a psychiatrist were responsible for the death of 25-year-old Leo Liu. During a clinical trial for Risperdal, Liu died of a heart injury that was “further complicated” by the drug and ignored by the study doctors. Janssen was found 70% responsible for Liu’s death and ordered to pay $5.6 million to the family.
Bernie Sanders Opposes Califf for FDA Post Cites Industry Ties
Bernie Sanders joins numerous public health groups and opposes Robert Califf's nomination to lead the FDA over industry ties.
Nominee to Lead FDA Removed Name From Recent Publications
Sheila Kaplan for the Boston Globe reports that Dr. Robert Califf, the Obama administration's nominee to lead the Food and Drug Administration (FDA), has removed his name from a series of scientific papers that he recently coauthored. The decision to remove his name, against publication ethics standards, has brought Califf under renewed criticism.
“The Life of a Professional Guinea Pig”
In the Atlantic, Cari Romm describes “what it is like to earn a living as a research subject in clinical trials.” “Phase 1 trials are almost always where the money is,” she writes, but they are “also the least regulated” and “companies aren’t legally required to register a trial with Clinicaltrials.gov.” “It seems to me like if you were considering signing up for one of these things, you would at least want to know the data that’s out there about [safety],” said Carl Elliott, an author for MIA and expert on the ethics of human subject research.
“New Depression Meds Not Effective Generally, But Drug Companies Insist Otherwise:...
The International Business Times covers a new study showing “trials for new antidepressant medications may not be applicable to the population at large.” “The finding, published in the Mayo Clinic Proceedings, shows recent trials are less generalizable than the prior studies, as researchers excluded most depressed patients from drug company-sponsored treatment studies.”
Are You Ready for Multiple Lawsuits By Victims of Psychiatric Misconduct?
Professor Leigh Turner of the University of Minnesota Center for Bioethics blasts the Board of Regents for ignoring psychiatric research abuse.
As Lawyers and Bureaucrats Delay, The Body Count Rises
It took over twenty years for the state medical board to sanction a Minnesota psychiatrist who was responsible for the deaths and injuries of 46 patients. Today, in the Markingson case, it looks as if history is repeating itself. How many patients die while bureaucrats delay?
And That’s the News from the Department of Psychiatry
In the business of clinical trials, the most valuable commodities are the research subjects. Filling clinical trials is hard, and filling them quickly is even harder. That’s why in 2000 a clinical investigator told the HHS Office of the Inspector General that research sponsors were looking for three things from research sites: “No. 1—rapid enrollment. No. 2 — rapid enrollment. No. 3 — rapid enrollment.”
The Road to Perdition
The recent research scandals out of the University of Minnesota’s Department of Psychiatry may be alarming, but they are not new. Back in the 1990s, when the university was working its way towards a crippling probation by the National Institutes of Health (for yet another episode of misconduct (this time in the Department of Surgery), the Department of Psychiatry hosted two spectacular cases of research wrongdoing, both of which resulted in faculty members being disqualified from conducting research by the FDA.
Fact-Checking the General Counsel in the Markingson Case
Ever since critics began asking questions about the death of Dan Markinson in a clinical trial at the University of Minnesota, the General Counsel for the university, Mark Rotenberg, has responded with a uniform message: the case has already been investigated many times, and no wrongdoing has ever been found. That's how Rotenberg responded to my article about the case in Mother Jones, and that's how he responded last week to the news that the Board of Social Work had issued a “corrective action” to the study coordinator for the clinical trial in which Markingson died.
The University of Minnesota was not Involved? Some Further Thoughts...
The suicide of Dan Markingson at the University of Minnesota has brought notoriety to the CAFÉ study and its site investigators, Stephen Olson and Charles Schulz. But the “corrective action” recently issued by the Minnesota Board of Social Work against the CAFÉ study coordinator, Jean Kenney, has raised another disturbing question.