Ioannidis Questions Strength of Psychology and Neuroscience Literature
Last week, well-known Stanford scientist John Ioannidis and his colleague Denes Szucs released a new analysis online. They examined research published in eighteen prominent...
Has Evidence Based Medicine Been Hijacked?
John Ioannidis claims that the idea of evidence based medicine has been “hijacked to serve agendas different from what it was originally aimed for,” in a newly published critical essay in the Journal of Clinical Epidemiology. Ioannidis frames the essay as a continuation of a conversation with David Sackett, widely considered the founder of evidence based medicine.
Treatment Guidelines Should Not Be Written by Professional Societies and Insiders
John Ioannidis, a leading expert on research methods, takes a critical look at the way professional societies write treatment guidelines.
New Study Concludes that Antidepressants are “Largely Ineffective and Potentially Harmful”
A new study published in Frontiers in Psychiatry concludes that “antidepressants are largely ineffective and potentially harmful.”
Researchers Test Harms and Benefits of Long Term Antipsychotic Use
Researchers from the City College of New York and Columbia University published a study this month testing the hypothesis that people diagnosed with schizophrenia treated long-term with antipsychotic drugs have worse outcomes than patients with no exposure to these drugs. They concluded that there is not a sufficient evidence base for the standard practice of long-term use of antipsychotic medications.
Researchers Expose Pharmaceutical Industry Misconduct and Corruption
Corruption of pharmaceutical industry sponsored clinical trials identified as a “major obstacle” facing evidence-based medicine.
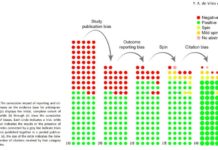
Multiple Researchers Examining the Same Data Find Very Different Results
A new study demonstrates how the choice of statistical techniques when examining data plays a large role in scientific outcomes.
Brain Scans Cannot Differentiate Between Mental Health Conditions
A new study analyzing over 21,000 participants found that differences in activation of brain regions in different psychological “disorders” may have been overestimated, and confirms that there is still no brain scan capable of diagnosing a mental health concern.
Researchers Set the Record Straight on Controversial Zoloft Study
An issue of Lancet Psychiatry is devoted to clarifying the lack of efficacy for Zoloft (sertraline).
Lancet Psychiatry’s Controversial ADHD Study: Errors, Criticism, and Responses
Amid calls for a retraction, Lancet Psychiatry publishes articles criticizing the original finding and a response from the authors.
It is Time to Abandon the Candidate-Gene Approach to Depression
The candidate-gene approach to depression goes unsupported and is likely based on bad science, new research finds.
Most Psychology Research Does Not Generalize to the Individual
A new study claims that quantitative research in psychology is “worryingly imprecise” and that generalizations may be flawed and misleading.
Withdrawal Symptoms Routinely Confound Findings of Psychiatric Drug Studies
Researchers examine how rapid discontinuation can mimic the relapse of mental health symptoms and confound psychiatric drug studies.
Biogen Pushes FDA to Approve Failed Alzheimer’s Drug
A new analysis, published in Lancet Neurology, demonstrates how Biogen is spinning results from two failed trials for a new Alzheimer's drug.
Study Examines the Difficulty of Withdrawing from Antidepressant Drugs
Correcting unnecessary long-term antidepressant use is difficult and met with apprehension by providers and service-users.
Safety Analysis Weighs Harms and Benefits of Antipsychotic Drugs
The researchers find that the drug effects for reducing psychosis are small and that treatment failure and severe side effects are common.
Reanalysis of STAR*D Study Suggests Overestimation of Antidepressant Efficacy
Reanalysis of the original primary outcome measure in the STAR*D study suggests STAR*D findings inflate improvement on antidepressant medication and exclusion criteria in conventional clinical trials results in overestimation of antidepressant efficacy.
Poor Evidence and Substantial Bias in Ritalin Studies
The authors of a large scale well-conducted systematic review of methylphenidate, also known as Ritalin, conclude that there is a lack of quality evidence for the drug’s effectiveness. Their research also revealed that Ritalin can cause sleep problems and decreased appetite in children.
New Review of Antipsychotics for Schizophrenia Questions Evidence for Long Term Use
A systematic review of the limited research available on the long-term effects of antipsychotics finds fewer symptoms in those off of the drugs.
Poor and Foster Care Children More Likely to be Diagnosed and Treated with Psychiatric...
Study details Medicaid-insured birth cohort’s exposure to psychiatric medications and mental health services.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Researchers Find Inadequate Reporting of the Dangers of Ketamine Treatment for Depression
Researchers report that dangerous side effects are not being adequately reported in the trials of ketamine for depression.
Review of Pediatric Antidepressant Studies Finds Evidence of Benefit Lacking
Review of pediatric antidepressant studies finds the vast majority are negative on primary outcomes and an increased risk for suicidality.
Suicide Rates Rise While Antidepressant Use Climbs
Multiple media sources are reporting on new data from the CDC revealing a substantial increase in the suicide rate in the United States between 1999...
Publication Bias Inflates Perceived Efficacy of Depression Treatments, Study Finds
Researchers report the cumulative effects of major biases on the apparent efficacy of antidepressant and psychotherapy treatments.