Tag: Study 329
Jon Jureidini–Evidence-Based Medicine in a Post-Truth World
In this interview, Jon Jureidini talks about the issues with evidence-based medicine and describes what led to the debasement of a system originally conceived to challenge extravagant claims and poor science.
Michael Hengartner – Evidence-biased Antidepressant Prescription
We talk with Dr. Michael Hengartner about his new book which addresses the overprescribing of antidepressant drugs and critically examines the scientific evidence on their efficacy and safety.
The Whistleblower and Penn: A Final Accounting of Study 352
After 18 years, the full story of the scientific corruption in a study of paroxetine for bipolar disorder, and the psychiatrist who blew the whistle.
Majority of Pediatric Antidepressant Industry Trials Considered Low Quality
Meta-analyses including studies that detail these trials could be presenting misleading information.
Farewell Mickey Nardo, 1 (not very) Boring Old Man
Mickey studied how the intimacy between leading academic psychiatrists and the pharmaceutical companies had impacted our profession. His blog was a treasure trove of analysis and information. Mickey did some heavy lifting, and for that we are all indebted.
Go Figure: Study 329
In the light of Study 329, is the consent that people or their families have given to take a medication like paroxetine any more valid than the consent that, after the event, an inebriated woman is claimed to have given?
Study 329 Taper Phase
Most doctors still affect surprise at the idea SSRIs might come with withdrawal problems. Regulators knew very clearly since 2002 about the problems, but have decided to leave any communication of these issues in company hands.
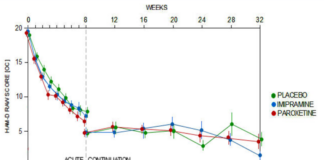
Study 329 Continuation Phase
All the fuss about Study 329 centers on its 8-week acute phase. But this study had a 24-week Continuation Phase that has never been published. Until Now.
Sadness: The Problem and The Solution
There is an ever-narrowing bandwidth of behavior that supports the dominant narrative in our culture today. We all need to act a certain way to protect the foundational beliefs of our time – that “science” has it all figured out, that rules keep us safe, and that it’s us vs. them (insert germs, terrorists, pests, and other “enemies”). But what are the consequences of this? What is this sadness and where does it go if we bandage our consciousness with business, medication, substances, or general avoidance of our real human experience?
Violence Caused by Antidepressants: An Update after Munich
The media is now reporting details about the 18-year-old who shot and killed nine and wounded many others before killing himself on July 22 in Munich. My clinical and forensic experience leads to a distinction among people who murder under the influence of psychiatric drugs. Those who kill only one or two people, or close family members, often have little or no history of mental disturbance and violent tendencies. The drug itself seems like the sole cause of the violent outburst. On the other hand, most of those who commit mass violence while taking psychiatric drugs often have a long history of mental disturbance and sometimes violence. For these people, the mental health system seems to have provoked increasing violence without recognizing the danger.
Interview: Researchers Deconstruct Ghostwritten Industry Trial for Antidepressant
Researchers, Jon Jureidini, Jay Amsterdam and Leemon McHenry, have taken a closer look at the data from a randomized control trial of citalopram (Celexa) that was ghostwritten and then used by the manufacturers to support claims of the drug’s efficacy and safety in the treatment of child and adolescent depression. To get the background on this story, we connected with Dr. Leemon McHenry, an investigator in this study and a lecturer in philosophy at California State University, Northridge.
Restoring Study 329: Letter to BMJ
When we set out to restore GSK’s misreported Study 329 of paroxetine for adolescent depression under the RIAT initiative, we had no idea of the magnitude of the task we were undertaking. After almost a year, we were relieved to finally complete a draft and submit it to the BMJ, who had earlier indicated an interest in publishing our restoration. But that was the beginning of another year of peer review that we believed went beyond enhancing our paper and became rather an interrogation of our honesty and integrity. Frankly, we were offended that our work was subject to such checks when papers submitted by pharmaceutical companies with fraud convictions are not.
Legal Journal Says Antidepressants Can Cause Violence and Suicide
Antidepressants have been reported to cause a state called “akathisia,” where people feel extremely agitated and restless and may become preoccupied with thoughts of...
“Anti-Depressants for Teens: A Second Look”
Writing for the Harvard Health Blog, Dr. Nandinia Mani reconsiders the use of antidepressants in teens in light of the reanalysis of Study 329. ...
“How Open Data Can Improve Medicine”
“Those who possess the data control the story.” In the wake of the reanalysis of the infamous Study 329, where scientific data claiming the antidepressant Paxil was safe and effective for teens was egregiously manipulated, researchers are pushing for open access to raw data. “The issue here, scientists argue, is that without independent confirmation, it becomes too easy to manipulate data.”
“A Decade of Questions over a Paxil Study Vindicated”
Martha Rosenberg calls the reanalysis of Paxil and Study 329 “a victory for safety activists, medical reporters, the public and freedom of the press.” But, she warns, “many pro-pill doctors continue to fight evidence of Paxil’s suicide risks and similar SSRIs.”
Is Motivation Worth More Than Expertise?
The strongest evidence we have as to whether a drug causes a problem does not come from RCTs or any other controlled study but rather from good clinical accounts. Even if RCTs were done by angels, so there was no hiding, no miscoding, nothing untoward, RCTs can still hide adverse events. The onus is on large and powerful corporations who have a lot of resources to pinpoint the populations where the benefit is likely to exceed the risk, if they want to continue to make money out of vulnerable people.
The Ghost of Research Future
Two facts about Robert Califf are beyond question. He is an expert on clinical trials, who is already seen as a leading architect of the future of medical research. And as the New York Times put it, he has “deeper ties to the pharmaceutical industry than any FDA commissioner in recent memory”. A lot of senior figures in medicine support Califf in spite of his ties to Pharma. The guy is just so bright, and understands the nuts and bolts of drug research so well! Surely a person like this is more useful than some outsider who offers only a squeaky-clean resume, they argue.
Study 329: Big Risk
Study 329 seems to fit the classic picture: It has Big Pharma ghostwriting articles, hiding data, corrupting the scientific process and leaving a trail of death, disability and grieving relatives in its wake. But is it at fault alone? Both Big Pharma and Big Risk (the insurance industry) were once our allies in keeping our hopes alive – in keeping our children alive and well. They are now a threat. And of the two – Big Risk is the bigger threat.
Study 329: Psychiatry’s Thalidomide Moment, Part 2
Nobody has retracted or apologized for a study that was an academic disgrace—but a marketing coup for GSK—which may well have caused untold numbers of deaths, suicide attempts and irreversible anguish to myriad families. Can we stand idly by when we’re told that it “accurately reflects the honestly-held views of the clinical investigator authors who do not agree that the article is false, fraudulent or misleading.”? What is the current market value of the honestly-held views of people who tell lies?
Study 329: Transparency in Limbo at the British Medical Journal
While making money from the publication of pharmaceutical company trials, and in the face of a complete failure by industry to adhere to basic scientific norms and make data available, BMJ and other journals — although BMJ in particular — have run a series of articles on supposed Academic Fraud. These articles feature instances of fraud sometimes as bizarre as researcher claiming he cannot show the data as it was eaten by termites. The universal feature is that these are academic studies, and academic fraud is an issue in academia.
Study 329: 50 Shades of Gray
Access to data is more important than access to information about conflicts of interest. It is only when there is access to the data that we can see if interests are conflicting and take that into account. Problems don’t get solved unless someone is motivated for some reason. We need the bias that pharmaceutical companies bring to bear in their defense of a product, along with the bias of those who might have been injured by a treatment. Both of these biases can distort the picture but it’s when people with differing points of view agree on what is right in front of their noses that we can begin to have some confidence about what we have.
Study 329: Conflicts of Interest
The BMJ states that it takes on average eight weeks from submission of an article to publication. The review process for Restoring Study 329 took a year, with a three-month review process involving six reviewers to begin with, and then a further four reviews in a four-month process, leading to a provisional acceptance in March that was withdrawn.
Study 329 in Japan
By 2002 GlaxoSmithKline had done 3 studies in children who were depressed and described all three to FDA as negative. As an old post on Bob Fiddaman’s blog reproduced here outlines, several years later they undertook another study in children in Japan. (Editor's note: This is a re-print, by David Healy, of a post by Bob Fiddaman)
Study 329: By the Standards of the Time
The controversy over “Study 329” on the effects of Paxil in teen depression has raised questions about the state of ALL medical research. I decided to look at the research for the most recent psychiatric drug approved by the FDA, a new antipsychotic called cariprazine or Vraylar. I located twenty studies of Vraylar on www.ClinicalTrials.gov, the U.S. government-sponsored registry for clinical trials. Three were still in process, and seventeen were completed. Not one had shared its results on the government website, a supposedly mandatory step.