A new review, published in Ethical Human Psychology and Psychiatry, re-assesses studies that compare electroconvulsive therapy (ECT) with placebo treatment for depression. The analysis also assesses the only five available meta-analyses that claim that ECT is effective.
The authors of the review point to the lack of quality research available to support the use of ECT, and call for the use of ECT to be suspended immediately until this research is done. John Read, the lead author, and Professor of Clinical Psychology at the University of East London, explains:
“In conjunction with the high risk of brain damage from ECT, this absence of efficacy evidence means that the cost-benefit ratio is so appalling that there is no place for ECT in evidence-based medicine.”
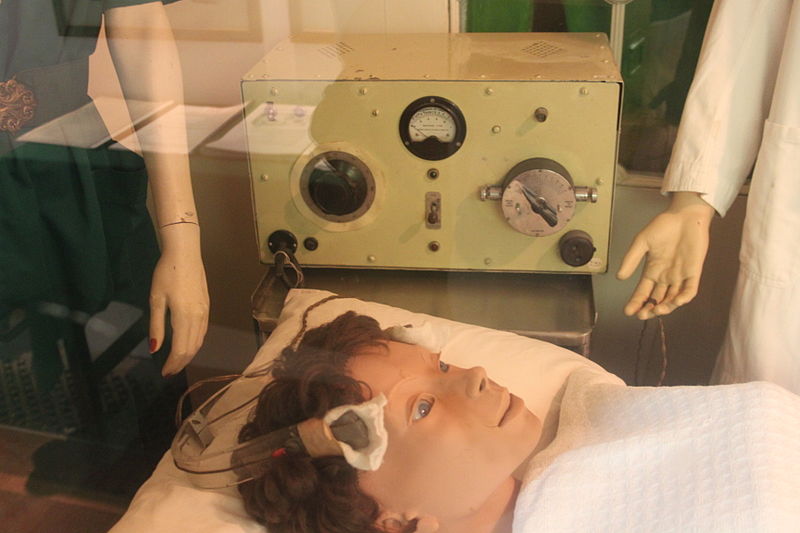
ECT is still administered to about a million people each year. ECT is used most often with older women as well as the severely depressed and suicidal. Two-thirds of those who receive ECT are women, and the average age of ECT recipients is between 60 and 65. Presently, ECT is promoted for treating depression when psychiatric drugs have previously been ineffective.
In the current review, the authors examined the quality of 11 studies that compared ECT with placebo treatment and five meta-analyses that examine these studies. In their assessment of the quality of each study, the authors rated the influence of bias on the outcomes of the research, and the quality of the research design and reports. The 11 studies have a mean Quality score of 12.3 out of 24 on a Quality scale developed and used by researchers for the review.
At first glance, the available research is extremely dated, with the most recent study taking place in the United Kingdom in 1985. The most recent study comparing ECT with placebo treatment in the United States is 57 years old. There has been no other comparable research conducted outside of the UK and the US. In a press release, Read states:
“This body of research is of the lowest quality of any I have seen in my 40-year career.”
The authors point to significant issues with how the studies reviewed were conducted. They highlight that none of the 11 studies are double-blind, meaning that neither the participants nor the researchers know who is in the treatment group versus the placebo group. Double-blind studies are crucial to avoiding mistaking actual change – in this case, reduced depressive symptoms – for what is, in reality, the placebo effect. Participants who have received ECT in the past would know that it is followed by headaches and temporary confusion, so, therefore, they would know whether they were placed in the ECT or the placebo group, invalidating the study.
Professor Irving Kirsch, Associate Director of Placebo Studies at Harvard Medical School, and the second author of this study added:
“I don’t think many ECT advocates understand just how strong placebo effects are for a major procedure like ECT.”
Irving Kirsch is a renowned placebo researcher, well-known for his landmark work investigating how placebo effects influence the effect of antidepressants, which was recently the focus of a follow-up study conducted by Polish researchers. To truly understand the impact of ECT as opposed to a placebo, it is crucial that studies are conducted in a rigorous manner – which is not the case with any of the studies that are available on ECT.
Moreover, although ECT is used primarily with women and the average age of recipients is between 60 and 65, only three of the studies had samples that were reflective of the demographics of ECT recipients. None of the studies examined the role of age or gender in their findings. None of the studies reported the ethnicity of the participants. Studies are supposed to be representative of the population demographics.
Further, although ECT is supposed to be given to severely depressed individuals as a last resort treatment, the majority of studies did not provide clear information to support that they included severely depressed participants. In fact, two studies used only moderately depressed patients, and one used participants with no depression diagnosis. This illuminates an apparent lack of evidence to support the use of ECT with severely depressed individuals. Kirsch explains:
“The failure to find any meaningful benefits in long-term benefits compared to placebo groups are particularly distressing. On the basis of the clinical trial data, ECT should not be used for depressed individuals.”
Other methodological concerns include the selective reporting of findings, small sample sizes, averaging only 37 people, a failure to examine the effect of ECT on the participants’ Quality of Life, and a failure to include participants who had tried antidepressants before the study. Less than half (46%) of participants had tried antidepressants before ECT – yet, ECT is supposed to be only used when all other treatments have failed, according to guidelines put in place by the National Institute of Clinical and Health Excellence (NICE).
The findings of the studies are unimpressive: four of the eleven found ECT significantly superior to Sham ECT at the end of treatment, five found no significant difference, and two found mixed results (including one where the psychiatrists reported a difference, but patients did not). Only two higher-quality studies report follow-up data. One produced a near-zero effect size in the direction of ECT, and the other a small effect size in favor of Sham ECT.
The lackluster results are concerning considering the damaging side effects of ECT, which can include permanent memory loss, brain damage, brain trauma, as well as death in a small number of cases.
Sadly, despite the risks of ECT, prior research by Read points to disturbing practices associated with ECT administration in England – highlighting its disproportionate use on older women, for persons outside of its target demographic, and outside of its evidence base, such as those diagnosed with personality disorders.
The meta-analyses examining these studies ignore the many of these limitations, and the majority of the meta-analyses are dated as well, with only one being conducted in the last 15 years. The researchers conclude that the major flaws in the study designs, small overall number of studies, and small sample sizes make it impossible to conclude that ECT is better than placebo in the short-term or long-term, or with its target demographic. Read has arrived at similar conclusions, citing the little to no evidence available to support ECT use in the short or long-term, or to treat depression or prevent suicide. The review concludes:
“The quality of most Sham ECT vs. ECT studies is so poor that the meta-analyses were wrong to conclude anything about efficacy, either during or beyond the treatment period. There is no evidence that ECT is effective for its target demographic – older women, or its target diagnostic group – severely depressed people, or for suicidal people, people who have unsuccessfully tried other treatments first, involuntary patients, or adolescents.”
Finally, the authors make a plea for the end of the use of ECT, arguing that the costs outweigh any potential benefits, considering that even the so-called benefits (reducing depression) of ECT have not been sufficiently validated by evidence:
“Given the high risk of permanent memory loss and the small mortality risk, this longstanding failure to determine whether or not ECT works means that its use should be immediately suspended.”
****
Read, J., Kirsch, I., & McGrath, L. (2020). Electroconvulsive therapy for depression: A review of the quality of ECT versus sham ECT trials and meta-analyses. Ethical Human Psychology and Psychiatry, 21(2), 1-40. (Link)















I smell a rat.
Perhaps “ethical” knows that most of the public would see this method of “treatment” as a tad unmedical, simply because any dummy would know you can’t isolate any parts of the brain, so that just those “diseased” parts get shocked.
The public likes chemicals better because you can be really creative and destructive with those, yet it’s just a little pill, like aspirin.
But then, chemicals do flood the brain too, don’t they? Or have the perfected the “treatments” to affect the “diseased” areas.
And some people REALLY dislike being forced to participate in biting on sticks, or swallowing chemicals, especially if they never hurt anyone.
Some people love to have the same rights as anyone. To learn, to make mistakes, to succeed or not. Some love the same legal rights, the same “standards of care”.
Some really don’t want a star on their arm.
Report comment
@ Sam,
Canada sounds horrific. Absolutely horrific. I hope some of you guys can move to the US. Hell, move to Mexico. It’s probably less of a dump than Canada when it comes to psychiatry. It seems like Canada is great for non-psychiatrised people. But once psychiatry is in the picture, it’s almost game over.
I don’t think I’ll ever visit that country. I don’t know how I’d prevent EMR from ever coming here. Maybe I should someday write an article about how devastating the EMR system is. It’s like suffering is not something to be helped, but to be punished. How did the world go so bat-shit insane?!
How did the developed world ever become so barbaric? And how do the clean streets and beautiful buildings and scenery even matter?
Report comment
Honestly, if one encounters manure, it smells the same everywhere.
Money talks and people have suffered forever by those who want more or think themselves to be the normal.
That is why it’s insane to try and fit in lol. Who actually wants to.
Report comment
“Presently, ECT is promoted for treating depression when psychiatric drugs have previously been ineffective.”
Thats the “stated aim”.
https://health.thewest.com.au/news/1212/shock-tactics-why-is-ect-feared
And with ‘advertising’ like this is it any wonder people are accepting their children being exposed to this “safe and effective treatment”. I think we will begin to see a reduction in the mean age of the recipients of this treatment. Many I would suggest will still be female, though they will be young teenage girls whose parents have concerns about their eating behaviours.
“Eat your greens Mary or we will have to plug you into the wall socket again”
Report comment
@boans:
Hey, how are you feeling? Is your partner out “of hospital.” Isn’t that how you say it?
I have to go back & read that long post oldhead sent to you. It was so kind of him
to do that. It looked really thorough.
You are still in my prayers! And your partner. Hope you are doing better!
Report comment
“this longstanding failure to determine whether or not ECT works means that its use should be immediately suspended.” I hope that happens. Thanks to John Read and Irving Kirsch for speaking the truth about psychiatry so consistently.
Report comment
While this may sound good on its face, “research” based on false premises — in this case that “depression” is a real “condition” to be “treated,” rather than a reaction to one’s life circumstances — is still flawed and invalid, even if the “conclusion” fits in with someone’s anti-ECT talking points.
Report comment
I wish I could rewind my life 15 years to the day I was sitting in the office of an esteemed Yale psychiatrist who, after having shocked my brain a number of times (he’d told me prior to my agreeing to the ECT that I was a “good candidate”, what with my “treatment resistant depression”; he’d assured me that his patients did not suffer the “side effect” of impaired memory.) explained why it was my fault I didn’t get better. What he told me in his office that day:. “You have borderline personality disorder. That’s why the ECT didn’t work.”. I wish I could go back in time. I’d throw this article in his face and never speak to another psychiatrist again. Instead I wasted another 15 years letting them gaslight me.
Report comment
The psychiatrists and psychologists are mostly gas-lighters, aren’t they? I also wish I could rewind my life 20 years, and knew the “doctors” were not trustworthy. Doctors today can be trusted, since most of them believe in the “bullshit” and “invalid” DSM belief system of the psychologists and psychiatrists.
Report comment
Someone Else,
Yes, they are gaslighters, in my experience too.
After I wrote that comment, I said to myself, if you could rewind why wouldn’t you rewind to *before* you had the ECT?
Maybe some residual damage from all the years of drugs and shocks lol.
I don’t understand why other doctors… internists, rheumatologists etc…stand with psychiatry as if it’s real medicine. You’re right, it makes them all untrustworthy.
Report comment
Because Katel,
“mental illness” was promoted like a cult. It explained the unexplainable. It promised to “help” or mostly “have knowledge” of. Of course it never “explained” “IT”, the reliance on psychiatry was simply a usable tool, and real medicine should be embarrassed to become desperate. They are all just to stubborn to say “I don’t know”… It is literally amazing that psychiatrists who one would hope are educated, still believe in this bunk, and I really don’t think that deep down they do believe it.
They can’t, logically.
It is just a belief of convenience, like many beliefs over eons.
If they honestly believed in it, there would be no use of harm towards people. NONE.
Harm has become the norm, not the exception. The harm starts at supporting someone in their thoughts that there is something “wrong” with them.
How horrible to do that to someone who is trying to weed through themselves, looking for wisdom in all the wrong places.
Psychiatry loves that process and likes to interrupt it.
One can only pass on what was experienced within the cult.
Report comment
Katel, in Canada,
one is marked for life. ALL of medical is involved. Because we cannot sue, it is obvious that the laws and governments are basically medical and psychiatric.
What other system works like this?
When there is no separation between “church and state”.
There is no separation between psychiatry and state, so we cannot “appeal” to “state”.
The only people we can appeal to, is the public.
People have to create their own church.
Katel, what you experienced is the norm in Canada. “co-ordinated with your psychiatrist”. This is a threat. It is saying, “I won’t give you medical care, because I think your pain is imagined”
It is also how they fix anyone that has chronic issues. It basically says “don’t bother us”.
Doctors HATE chronic stuff or suffering. Instead of telling you not to come back, they write lies, innuendos in charts.
I had an ER doc say to me “if anything bad was going to happen, it would have happened already” (verbatim) LOL.
THAT which he said, gets recorded on file, and THAT opinion gets used against me.
They showed me what I’m up against and what my options are.
I find it commendable that you decided to think of them as pathetic. And truly it is.
No they don’t cry themselves to sleep. Our society has created so much of a mess, and there are so many people, plus the fact that docs are sick of the mess that was created, yet each doc takes it out on the people.
It happens so often that it is simply everyday practice. You are FAR from the only one and that has desensitized them to the point of self righteousness.
They have become an angry lot. It is palpable.
They absolutely despise tears also.
Report comment
KateL,
The other doctors bought in for two basic reasons. The psychiatrists cover up the easily recognized iatrogenesis/malpractice, for the mainstream doctors. And the mainstream doctors eventually learned how insanely profitable all of the psychiatric industry’s iatrogenesis is, for the entire medical field. And what’s really sad to me is even the religions, who own the hospitals, bought in, because the psychiatric and psychological industries systemically cover up the “zipper troubles” of the pastors and their wealthy parishioners.
Thanks for understanding, and missing my type-o. Of course, I meant to say, “Doctors today can not be trusted ….”
Report comment
SE,
This makes sense. Sad, though…for them. I hope they cry themselves to sleep at night, or at least wake up with headaches.
I broke down and had an appointment with a rheumatologist a few weeks ago, by video chat. I wanted to get some blood work. If I could have ordered it myself I would have.
I thought it would be easier to do a video appointment than an in-person appointment but I still was crying by the end of our chat. This doctor, who was very nice and sympathetic to my issues, couldn’t understand why I had gone off Cymbalta because, she said, Cymbalta is a safe and effective drug, the only danger is a slightly higher risk of suicide when people first start taking it, because they feel so much better.
Yeah, she really said that. Unbeknownst to me she referred me to a pain doctor who’s office called me yesterday. I called back to schedule the appointment today and learned from the assistant that the pain doctor would only agree to have an appointment with me if it were coordinated with my psychiatrist. I asked why this was and the assistant said something vague about “issues in your medical records”. Yeah I’ve been blacklisted. Probably all the Google reviews I wrote. I explained to the assistant that I do not have a psychiatrist, that I will not have a psychiatrist, and that I am a psychiatric survivor and do not need an appointment with the pain doctor. I just got finished writing a Health Grades review of the pain doctor describing this experience.
I was so upset about this all day. Now I just think it’s stupid and kind of pathetic on their part. I have Kratom for pain and I don’t need a prescription for it so I don’t need a pain doctor anyway.
Report comment
Yeah, I always feel like killing myself when I feel better. What kind of nonsense is that?
Report comment
“…the only danger is a slightly higher risk of suicide when people first start taking it, because they feel so much better…”
I’ve heard this before – its just clever talk!
(I have never attempted suicide OFF medication).
Report comment
John Read and Irving Kirsch have debunked the paltry evidence for the efficacy of ECT. However orthodox psychiatry still promotes ECT largely because it supports the flawed belief that “mental illness” is a brain disease and should be “treated” by concussing the brain with electrical shocks three time weekly for several weeks typically.
Dr. Dainius Puras who is a psychiatrist and is the UN Special Rapporteur on the right to health in his 2017 report states :
“The history of psychiatry and mental health care is marked by egregious rights violations”
“We have been sold a myth that the best solutions for addressing mental health challenges are medications and other biomedical interventions.”
“For decades now, an evidence base informed by experiential and scientific research has been accumulating in support of psychosocial, recovery oriented services and support and non-coercive alternatives to existing services.”
Not uncommonly ECT is still given involuntarily without informed consent and mandated by regressive involuntary commitment laws. It is high time this procedure was declared redundant and legally banned. ECT should behold be cast into the archaeological history of psychiatry along with lobotomy, insulin coma, rotating chairs and wet packs.
Report comment
Most people would allow that electrocution and grand mal seizures would be things that anyone would want to avoid at any cost. It shows how utterly distorted the world of psychiatry is that opponents of “ECT” have to do anything at all to debunk the obvious harms that inducing a grand mal seizure would create. People who have near-death experiences often recover an appreciation for the fragility of life and make big changes to make their lives more meaningful. Does this suggest we should push people in front of cars as a form of “therapy?”
I also doubt the patient ratings are reliable. After 3-4 “treatments,” I’m guessing most of the “patients” are very much inclined to say, “I am feeling SO much better! I feel COMPLETELY cured! Now, can you please open that door and let me the hell out of this place?”
Report comment
“Does this suggest we should push people in front of cars as a form of “therapy?””
Hey I just realised that there’s no need to waste the chemicals for the Euthanaisia of “patients”. More savings for the taxpayer. Imagine the insanity of making killing a form of treatment. Medicalise anything these days and you can avoid any problems with the law, and in fact can be provided with assitance in your criminality.
Report comment
Ever see the movied, “What About Bob?” with Bill Murry and Richard Dreyfuss and Julie Hagarty?
“Death therapy, Bob! It’s a sure cure!”
Report comment
A reason people appear to be better is that they received brain damage and are cognitively impaired. It’s harder to articulate how shitty you feel when you’re unable to remember what occurred 10 minutes ago or articulate your pain.
Report comment
Ashley, thank you for contributing this very informative article! By modern scientific standards, Read and Kirsch are right, there is no scientific justification for using ECT. Psychiatry should be ashamed for deeming this procedure to be scientifically credible. I have always been extremely skeptical of ECT but never knew the evidence was this pathetic. I feel embarrassed because I should have known the full story about the science. Nobody teaches this stuff because articles like this – which speak the truth via critical scientific analysis – are so rare and don’t make it into the textbooks. And psychology training doesn’t touch on it hardly at all and we allow psychiatrists to pronounce what the evidence says. Thank you. Your article is one I won’t forget. I’ve already sent it to several clients.
I worked at the Mayo Clinic for two years. Psychiatrists there often described ECT as the most effective “treatment” for “depression” – by far. The “by far” was often emphasised, as if the evidence base was so solid as to be beyond reproach. They were so quick to recommend ECT to depressed clients. In fact, they routinely provided it to depressed teenagers. I once had a teenage client there who became depressed in the context of negative life events and had “failed to respond” to a trial of an “antidepressant” (which, research clearly shows, don’t work in adolescents and probably do more harm than good). He was then started on ECT. Never saw him again. I imagine thousands of other adolescents have been so “treated” by Mayo Clinic psychiatrists.
Note: Mayo Clinic endorses the chemical imbalance cause of depression, and the notion that “antidepressants” correct a chemical imbalance, on its website. The section listing causes of depression includes the following causes: “biological differences,” “brain chemistry,” “hormones,” and “inherited traits.” The psychiatrists who author its website just can’t bring themselves to acknowledge that negative life events can cause “depression.” I know those people, worked with them, and it doesn’t surprise me at all. Negative life events are merely “risk factors” to them, not causes. This pathetic obliviousness to scientific and common-sense reality fits my first-hand observations of the psychiatrists I worked with and shared clients with, and was formative in shaping my opposition to psychiatry’s invalid biomedical model.
Psychiatric organisations promote the myth that ECT is a last resort treatment, given only to older depressed people who have failed every possible alternative. This is a PR stunt and bears no resemblance to real-word practice. I see clients of all ages, with all manner of struggles, who are recommended ECT, or agree to ECT, or are forced to receive ECT, by psychiatrists who falsely portray this “treatment” as highly effective and safe, despite it being neither. I work in private practice now in Australia. I saw a client who received ECT at age 14. Last week, my 40-something client struggling with anxiety saw a psychiatrist for the first time who recommend ECT, TMS, and “antidepressants.”
Once again, this situation reveals the reality Bob Whitaker and Lisa Cosgrove laid bare in Psychiatry Under the Influence: the profession of psychiatry cannot be trusted to provide the public with accurate information about any of its “diagnoses” or “treatments.”
Ashley, thank you again for your work, it is most appreciated.
Report comment
“Moving cars are a “risk factor” for broken legs, but clearly, there is a biological vulnerability for people whose legs break when hit by moving cars, because after all, not ALL people who get hit by cars break their legs, so there MUST be something different about those “weak-legged people” who just can’t take the hit and keep on walking!”
Report comment
I have a follow-up comment. I Googled the following phrase: “[ECT] is the most effective treatment for depression” and what showed up is what I predicted. Psychiatry departments and psychiatry researchers often make this specific claim which, as Read, Kirsch, and McGrath laid bare, is complete bullshit. My Google search finds psychiatrists from countries around the world making this claim, often in peer-reviewed scientific journal articles, with no scientific justification, as if it is simply taken as a matter of faith. This fits my experience with Mayo Clinic psychiatrists. Obviously, belief in the safety and effectiveness of ECT without regard to the actual research is a faith-based pillar of the profession. A few examples (of many):
https://www.dartmouth-hitchcock.org/psychiatry/electroconvulsive-therapy-ect.html)
https://www.biologicalpsychiatryjournal.com/article/S0006-3223%2803%2901046-1/abstract
http://www.irishhealth.com/article.html?id=8801
Report comment
Hello.
10 years ago I went through 13 ECT treatments after my meds stopped working and I was suicidal. I was told that ECT was my last hope because nothing else was working for me. I was inpatient at the time and desperate to try anything in order to get out.
I can honestly say that it worked and saved my life. My meds worked again and I had returned from a crippling state of depression. The only reason treatment was stopped was because my memory was becoming fried. I lost so much, I can’t even begin to tell you. The worst had to be losing all memory of my son’s birth up until his 1st birthday.
10 years later I still have serious memory problems. It detrimentally affects my everyday life not to mention the havoc it creates at work. My question is, has anyone come up with a way to help people like me? I understand ECT causes brain damage and that there’s not much that can be done. This is just a shot in the dark but has anyone heard of someway to repair the damage?
Memory loss naturally gets worse with age and it’s making things even worse for me now.
Thank you in advance.
Report comment
Maybe the first thing to consider is that the ‘meds’ made you suicidal in the first place by you becoming toxic on them. Would you consider that or no way ?
Report comment
Hi pleasehelp;
It has taken me too long to reply. I woke up tonight realizing that I have given condolences to another ECT survivor further down without giving the same regard to you. That’s not okay and I am sorry.
I had ECT and lost memories also. The memories never returned, but I have relearned some of the skills lost with time and effort. I continue to experiment with nutrition and supplements, but I haven’t found a fix. Enjoy your family and have fun as much as you can. I find new memories are healing.
🙂
Report comment
Please help,
I attended a Maudsley debate where Dr John Read debated, alongside a very genuine Lady doctor who had been seriously damaged by ECT. I believe she attested that she did make a recovery through psychological means. But I don’t remember this Lady’s name off hand.
Report comment
Sam,
Thanks for your comment. As painful as it to hear that you have also experienced this level of discrimination and intimidation from people in the “helping” professions, it’s also validating in that it reminds me that it’s not in my head, I’m not paranoid. This is really happening to me and countless others. I had hired a patient advocate and told her a bit of my history and I emailed her to let her know about what happened with the pain doctor. She wrote back saying, “I’m sorry you got bounced around today,…but this is the system and we can’t change the system. I recommend that you reinstate your appointment with the rheumatologist.”. I didn’t get bounced around. I got discriminated against, based on my record, my medical record, my medical rap sheet… As far as these people are concerned. Yes you’re right they hate tears. They also hate anger and hopelessness and being contradicted. They hate everything except robotic agreement that the ridiculous things they are saying actually make sense.apparently they can’t handle anything except a doctor knows best attitude even when they’ve proven over and over again that they know nothing.
Report comment
We can’t change the system? Really? We ARE the system! What an apathetic approach she is promoting!
Report comment
Yes, Steve. It’s like a verbal shrug. No thanks. She said, “you’re self medicating (with Kratom) and that has it’s risks.”. I told her I will continue to self medicate, that being my safest option. I really don’t need to hear more from the rheumatologist about how safe Cymbalta is as long as it doesn’t make me feel so good that I want to…you know. I don’t need to hear anything else from any of these people. Imagine how much more money they would make off of me if I listened to them though. That’s a rheumatologist, a pain specialist, a psychiatrist to, you know, coordinate my appointments with the pain specialist, a primary care doctor which they keep telling me I need…and then the stay in the hospital when they all drive me over the edge.
Report comment
It is a dead end street in Canada. And basically global if you fall into the wrong hands or situation.
People protesting here and saying “defund the police” and “fund “mental health” instead”
People really have no clue until experienced, and I get that, but at a certain age, people should listen when others are talking about abuse. The people are not going to claim abuse unless something is indeed going on.
Report comment
“We can’t change the system” — exactly our argument for anti-p.
You can rearrange the drapes and potted plants though, that has to count for something.
Report comment
Katel, it’s NOT YOU. Well you knew that, but it is still hurtful when you know something is very wrong and that it happens to so many.
I spent 3000 on an advocate in another province, because I knew that our government’s advocates are PR FOR the system.
Even the 3000 got me nowhere. It was really a nurse from the US I happened to meet on a forum that told me to stop paying them.
Even doctors hate the system. They hate it because they created a miserable place. I don’t think many realize just how ugly they can make it for you.
Report comment
Also, Sam, “if something bad were going to happen it would have happened already”? What does that even mean… That you were supposed to show gratitude because you had been spared some unnamed abuse? Ridiculous the things they get away with.
Report comment
Well I was there with my breathing issues once again, so this was the pulmunologist’s way of telling me that it was my “fear” that brought me there. And his attempt at reverse psychology plus it was a way of saying “don’t come back”.
They never bothered to check that there were two times in 60 years I had been to ER and both were emergency surgery after begging and having to prove myself.
They made me realize where I do not want to meet my maker. They also met their match.
“I will not back down when pushed too far”
Report comment
As if there were something wrong with coming to the ER because you “fear” there is something wrong. Unbelievable how they twist everything.
Sam, I just want you to know I draw a lot of strength from reading your comments. Thank you for being here.
Report comment
Katel,
It is good to hear that you benefit from reading. It is important to be sure within yourself.
It is so blatantly obvious that their collective dealing with people really has ZERO to do with the person, everything to do with them.
It is an ill system based on power and protection of self/job.
The “standards of care” was specifically developed for this.
I have looked at them in a kind of amazement. I used to get shocked, but now it’s more about observing. So I can see how shrinks became shrinks.
It’s the EASIEST job to “diagnose”. Children do it automatically.
Report comment
Haven’t posted for a long time. My husband died. He was the custodian of my memory. I’m missing 15 to 20 years of my life – including most of my marriage. My husband’s heart failed when he realized what had happened to me after he was coerced into signing consent forms for ECT – I had refused. I became his caregiver for 15 years until he died with my hand on his shoulder. His doctors had medicated him to death – he could not metabolize any of the drugs administered. Me? Why did I have ECT? No one recognized that I was sensitive or allergic to benzodiazepines and dozens – yes, dozens – of drugs were given to me in a few months – until I was considered ‘an excellent candidate for ECT.’ My soul died and my husband’s heart failed when we were told 1. I was demented 2. I would not be going/coming home, but rather to an institution 3. I would be taking pharmaceuticals for the rest of my life 4. I would need maintenance ECT for the rest of my life. There was nothing wrong with me. All pill bottles should have the word ‘POISON’ written on them. Why did I take the benzo? Insomnia from caregiver stress while my mother was dying. I cold-turkeyed off the benzo. I tapered myself off all drugs and stopped going for ECT. I am damaged in many ways.
Report comment
🙁 Not OKAY! Sorry is all I have but it’s just another insult after everything you’ve lived.
When will testimony be evidence enough? How many eye witnesses does society need?
Report comment
Amnesia,
I am so sorry. I wish I had something useful to say to you. All I can say is, I believe everything that you say and I am so sorry that these things happened to you. I can’t imagine how strong you must be to have survived all of it. All of us who have survived these atrocities are stronger than anyone “looking in from the outside” gives us credit for.
Report comment
Welcome back Amnesia.
You are here and you remember enough and have insight enough to know what happened to you. I doubt too many shrinks could hold up under what was done to you.
Are they ashamed? Doubt it.
I bet they wonder what it must be like to have concern about others. They use pills and a mass murderer a gun. A gun kills far fewer people.
Do those in legislative places think I’m exaggerating? I doubt it. They don’t want to discover the reality. It’s too broken and psych is too powerful.
What I know from history is that dynasty does not last forever. Beliefs change. Perhaps they will play with genetics and breed themselves out.
Who knows. Perhaps citizens across the world will unite and become a huge issue.
Report comment
Wow. No words.
Report comment
@amnesia:
I am SO SO sorry you lost your LOVE! And for all
you’ve endured. : ( : ( : (
Report comment
My husband came every day. He came in the morning after ECT to tell me where I was and why I was being tortured. And he wiped the blood from the corners of my mouth. He came three days a week for 2 1/2 months – sometimes twice a day. After seeing me he drove to the other side of Toronto for work – at lunch he shopped for groceries – after work he went home and cooked a meal for me – he made enough so I would have leftovers for lunch the next day. Then he came to see me with supper. The next morning he was back. When he collapsed, I quit ECT. Fifteen years later I still have tremors, vision problems, breathing problems. I can’t walk properly and I can’t run at all. And now I am alone. A recent CT scan shows some small white spots on my brain – I wonder if those are what remains after the brain bleeds – petechiae. Will update you. I cry a lot. There seems to be no purpose to life – other than revenge. I am obviously not demented, not in an institution, not receiving pharmaceuticals nor maintenance ECT for the rest of my life. THEY were wrong.
Report comment
I’m sorry for what they did. I’m sorry that there are people, it used to be only men, which tells us that the “men” who did these things, had no room for women to do these things. Then a brand of feminism was born, which was not about protecting other women from Electroshock or being labeled…. but rather how to join the ranks of the men “treaters”.
After all, we live in a politically correct time, where a woman needs to be present for services delivered to women. Why women considered it to be feminism to join legal abuse.
I cannot use the title of “doctor” for a certificate received from hoax school.
Report comment
amnesia:
I am planning a break from MIA & still plan on it, but I caught both this post & Rosalee’s recent post on the George Floyd blog & I cannot leave until I say something about both of them.
O.O. said sorry is an insult after all you’ve been through.
oldhead said, “wow. no words.”
`cause all of us on here know that we have to say something, even though there is nothing to say….
“There seems to be no purpose in life–other than revenge.”
That’s how I feel too. Channeled rage (even though the criminals that stalk me think I am going to harm myself or others–they are wrong) is how I streamline my story.
I wish we could set up a memorial for your husband on this site. I don’t know what that would look like. I know it’s not enough. NOTHING is enough & it never will be.
ABOLISH PSYCHIATRY!
How are you doing today????
Report comment
Snowyowl,
Thank you.
Report comment
Thank you for this! I had been unaware of his study, even though it seems it was published over 6 months ago. It only confirms what we already knew, but I’m glad it did, as these are people with academic credentials.
In spite of the authors’ desire for “better data” it seems the results of these studies would indicate that it would in fact be unethical to do a double-blind study or anything like that on ECT. The drug studies are bad enough. Of course, if psychiatrists would agree to be the study subjects (they wouldn’t)…I might be persuaded to look favorably on that particular study.
Report comment
Thank you for this important article, which I hope people will read with Dr. Lauren Tenney’s recent, devastating article about electroshock that was also published in MadInAmerica!
I do wish that EVERYONE would stop calling it ECT, as the late Leonard Roy Frank — himself a victim of electroshock — used to plead with people to do, because, as he pointed out, the “T” stands for “therapy,” which is dead wrong for something that wipes out people’s memories, as they did to him. Also, to say “ECT” means not coming right out and saying “electroshock.” Even the “C” in “ECT” is a bit of whitewashing, since it stands for “convulsive,” which sounds more clinical and less horrible than “shock” or “seizures.” We can all help educate people about what this involves, and one of the ways to do this is always to call it “electroshock.” Make people hear those words and know what it is.
Report comment
I agree Paula.
Report comment
I also agree…
Report comment