Published reports of clinical trials of psychiatric drugs typically include a graphic showing the efficacy of the study drug in reducing symptoms of the disorder compared with placebo. These graphics are visually compelling. They almost always show a notable separation between the study drug and placebo in the reduction of symptoms over time, and thus the reader can see what the authors of the study conclude: The study drug, at one dose or another, is an effective treatment for the disorder.
These graphics, however, are presenting an illusion of efficacy. It’s done very simply: The graphics are set up with a vertical axis that, in essence, acts as a magnifying glass.
In a recent MIA Report titled Anatomy of an Industry: Commerce, Payments to Psychiatrists, and Betrayal of the Public Good, Mad in America looked at the financial influences present as seven new psychotropics were brought to market from 2013 through 2017: four antipsychotics, one antidepressant, and two drugs for tardive dyskinesia. The published reports of the pivotal trials of these drugs regularly featured a graphical illusion of this sort, an illusion that can be revealed by re-graphing the efficacy data with a proper vertical axis.
Here are the graphics that tell of this illusion of efficacy for each of the seven drugs, along with similar graphics for a fifth antipsychotic that was approved in 2020 (lumateperone.)
Oral antipsychotics as a treatment for schizophrenia
In pivotal trials of a drug being tested as a treatment for schizophrenia, the primary outcome measurement is reduction of symptoms on the Positive and Negative Syndrome Scale (PANSS). The scale is composed of 30 questions, with each answer scored from 1 to 7, and thus it is a 210-point scale (with possible scores ranging from 30 to 210.) However, the efficacy graphics in published reports do not use that range of possible scores as a vertical axis; instead, they regularly use a 25-to-30 point segment of the PANSS scale, which acts like a magnifying glass in presenting the “separation” between drug and placebo.
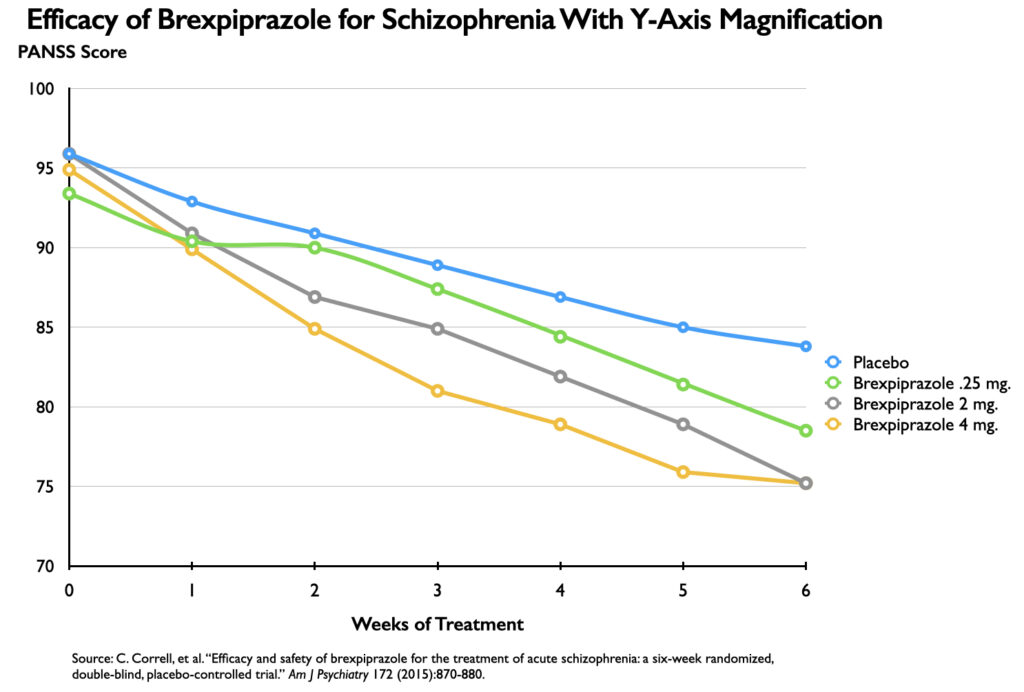
Rexulti/brexpiprazole
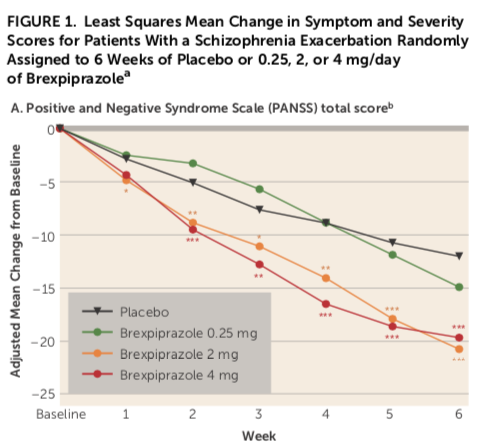
In a phase III study published in 2015, three doses of brexpiprazole were compared with placebo over a six-week period. Here is the graphic that told of the drug’s efficacy at these three doses:
It appears that there is a significantly different course for those treated with brexpiprazole compared with placebo. However, the vertical axis tells of a reduction in symptoms from 0 to 25 points on the PANSS scale. That’s the magnifying effect: There is no information in this graphic that tells that this point drop occurred on a 210-point scale. The use of a 25-point segment of the scale, instead of one with scores spanning 180 points, could be said to magnify the difference by a factor of seven (180/25).
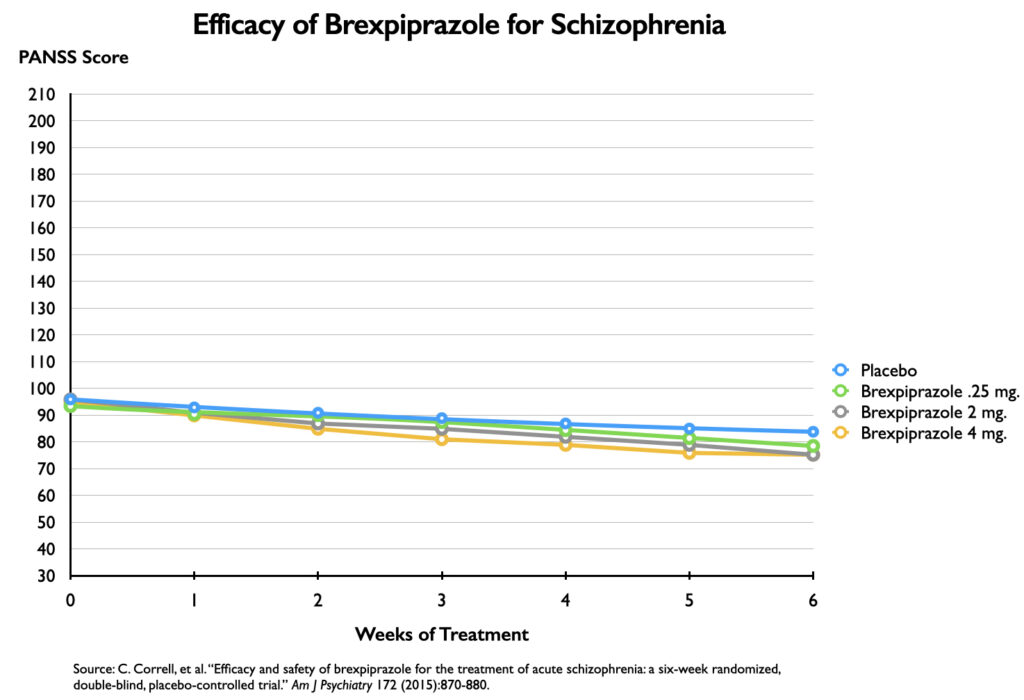
The proper perspective would be a graphic that charted PANSS scores with a vertical axis of 30 to 210. This would depict the clinical course of the four groups over the six weeks, and it would reveal whether there was a significant difference between the placebo and brexpiprazole cohorts.
As can be seen in this graphic, there is almost no difference in the clinical status of the four groups at any time during the six weeks. Researchers have determined that there needs to be at least a 15-point difference in PANSS scores between drug and placebo for the treatment to provide a clinically important benefit, and none of the three brexpiprazole doses met that standard.
This graphic also reveals that “statistical significance” shouldn’t be confused with clinical significance. Although the outcomes for the three brexpiprazole cohorts seem indistinguishable, two of the three doses—the 2 mg and 4 mg doses—squeaked over the “statistically significant” line, and this led the study authors to conclude that the drug was safe and effective at these doses.
The first graphic above charted a drop in symptoms; the second graphic charted the PANSS scores. It is possible to re-apply the magnifying effect to the PANSS scores by replacing the 30-to-210 point axis with one that runs from 70 to 95 points. Just as in the first graphic, a notable separation between drug and placebo now appears. There is the same seven-fold magnification of the results.
The “safe and effective” finding in a published article, together with the visual that tells of a notable separation in symptoms between drug and placebo, provides the “evidence” that a drug manufacturer can use to promote its product. The manufacturer pays psychiatrists to serve as its advisors, consultants, and speakers, and collectively this group authors the trial findings, writes further reviews of the drug, and speaks about its usefulness at dinners, conferences, and CME webinars.
That promotion turned Rexulti into a commercially successful drug, one that generated $1.4 billion in Medicare and Medicaid sales from 2015 through 2019.
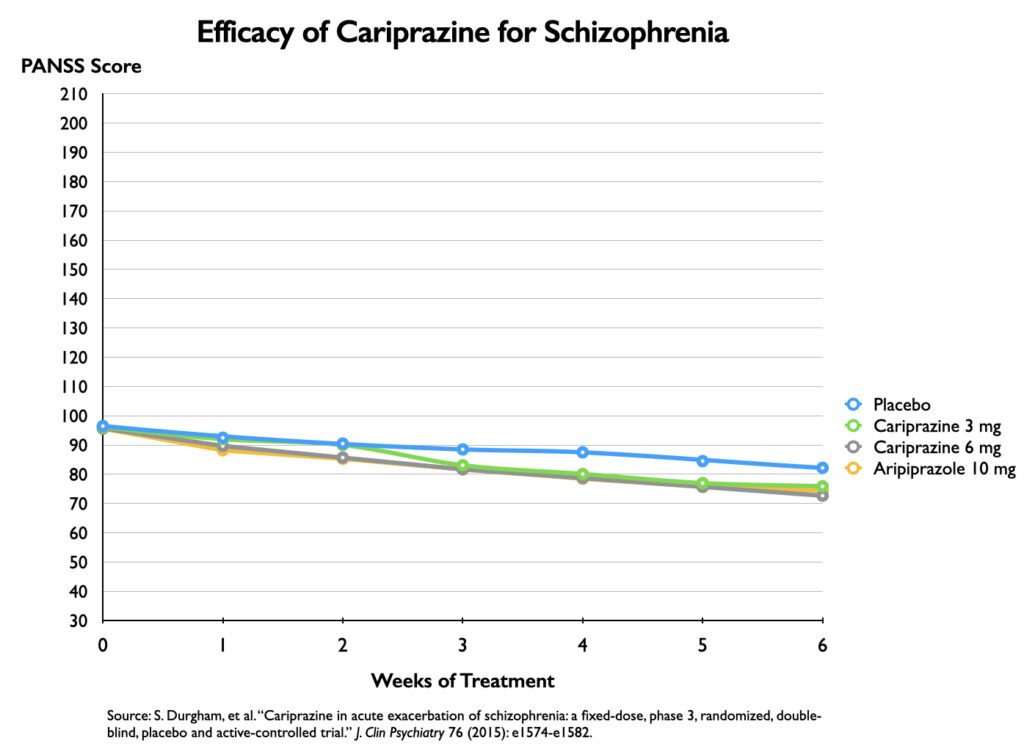
Vraylar/cariprazine
In a phase III trial of cariprazine, which was published in 2015, two doses of cariprazine were compared with placebo and to a 10 mg dose of aripiprazole. The efficacy graphic that was published used the same 25-point vertical axis showing a drop in symptoms as the brexpiprazole publication did.
The graphic shows a notable separation in symptoms between the placebo group and the three groups treated with cariprazine or aripiprazole, with this separation becoming apparent at week two and more pronounced over time.
Now here is a graphic that charts their PANSS scores over time:
Once again, the lack of clinical significance is evident in this visual. The graphic provides the same understanding that the numerical data does. At the end of the study, there was only a 10-point difference between placebo and the 6 mg dose of cariprazine, and the drug-placebo difference was even less than that for the 3 mg dose. Both the graphic and the data tell of a drug treatment that did not provide a meaningful clinical benefit.
However, the small differences in PANSS scores were “statistically significant,” and thus the authors concluded that cariprazine was safe and effective at both 3 mg and 6 mg. The usual promotional machinery swung into gear once the drug was approved, and yet another billion-dollar drug was born. Medicare and Medicaid sales of Vraylar totaled $1.2 billion from 2016 through 2019.
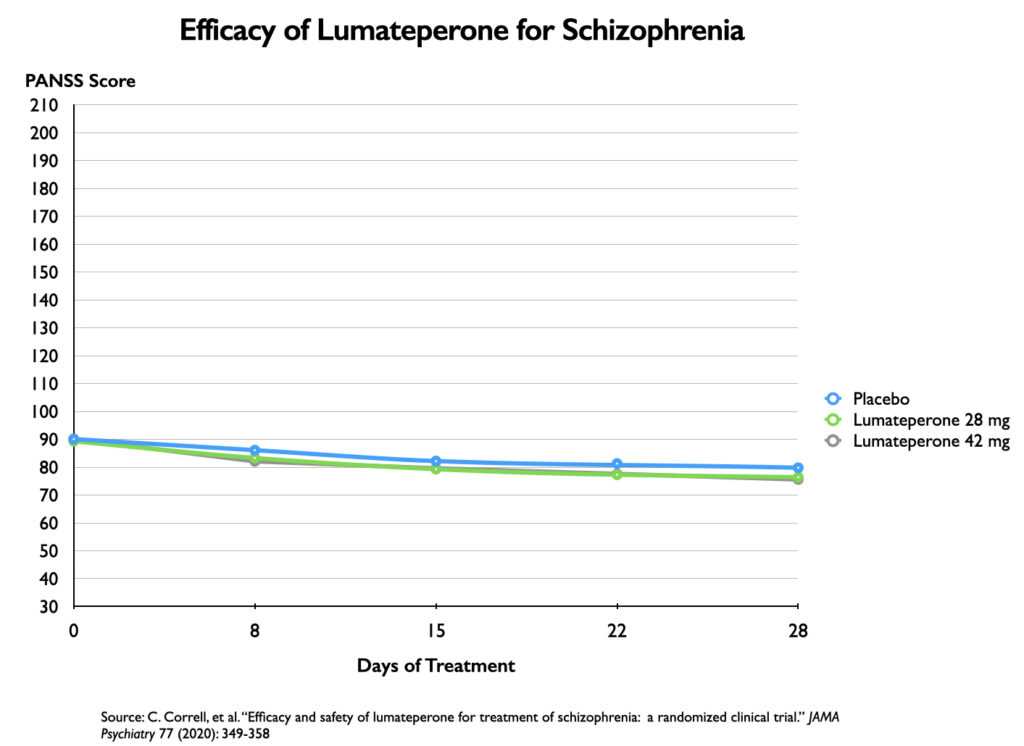
Caplyta/lumateperone
A phase III trial of lumateperone, published in 2020, compared two doses of lumateperone with placebo over a four-week period. The efficacy graphic used a 16-point vertical axis, which ramped up the magnifying effect 11-fold.
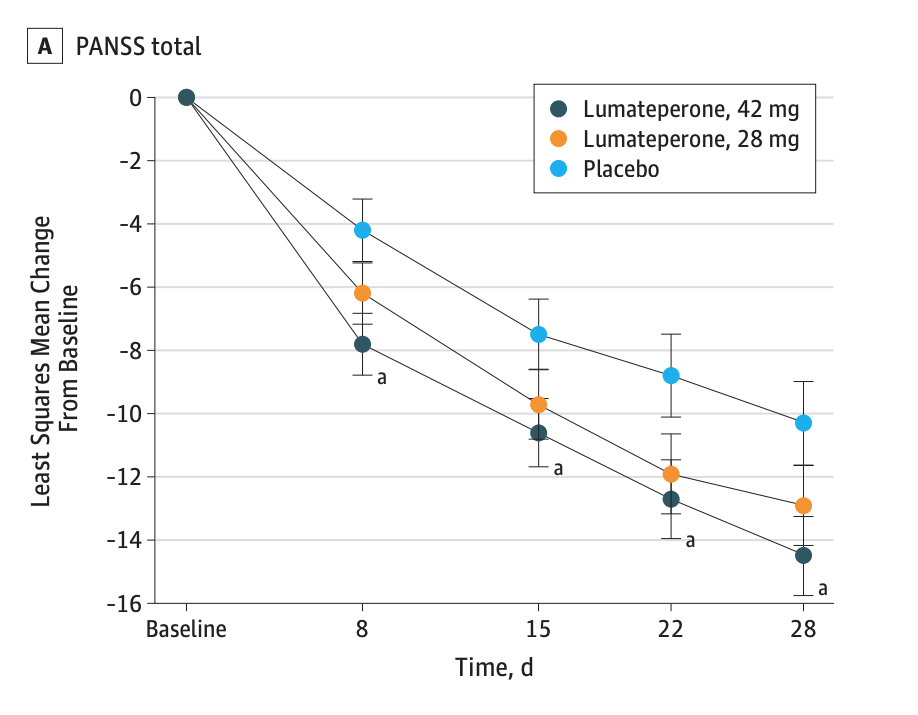 Once again, it appears that there is a significant difference in the decrease in symptoms on PANSS for the two medicated groups compared with placebo. Here is a graphic of their PANSS scores over the four-week period:
Once again, it appears that there is a significant difference in the decrease in symptoms on PANSS for the two medicated groups compared with placebo. Here is a graphic of their PANSS scores over the four-week period:
There is virtually no separation between the medicated and placebo groups in this graphic. In fact, there was only a 3.2-point difference in PANSS scores at day 28 between placebo and the 42 mg dose, and a 2.4-point difference between placebo and the 24 mg dose. These differences, on a 210-point scale, were clinically meaningless, and yet the 42 mg dose was deemed to provide a statistically significant benefit.
Equally revealing, the 24 mg dose did not pass the “statistically significant” hurdle. As there was a slight difference in the baseline scores for the two doses, the total reduction in symptoms for the 42 mg dose was 1.6 points greater than for the 24 mg dose, and in the world of statistical significance, that miniscule difference separated an “effective” drug from a “non-effective” one.
As lumateperone did not reach the market until 2020, there is no public record yet of Medicaid and Medicare sales of Caplyta.
Injectable antipsychotics as a treatment for schizophrenia
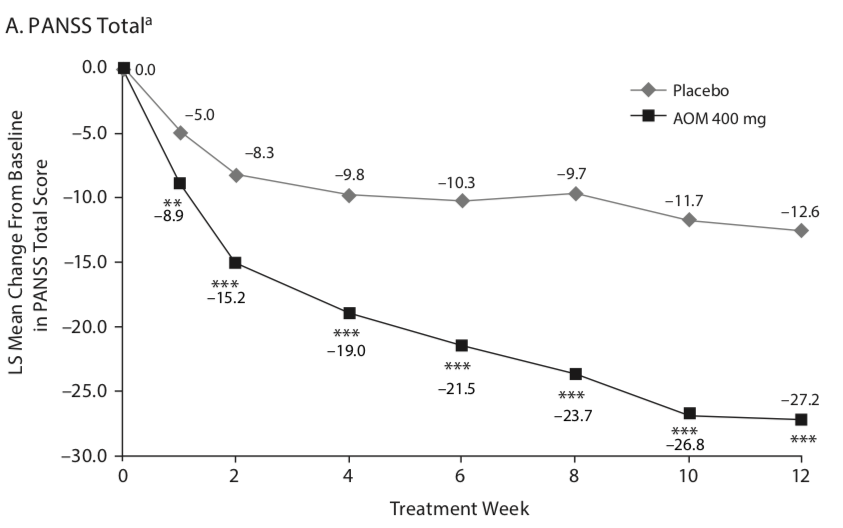
Abilify Maintena/aripiprazole once monthly
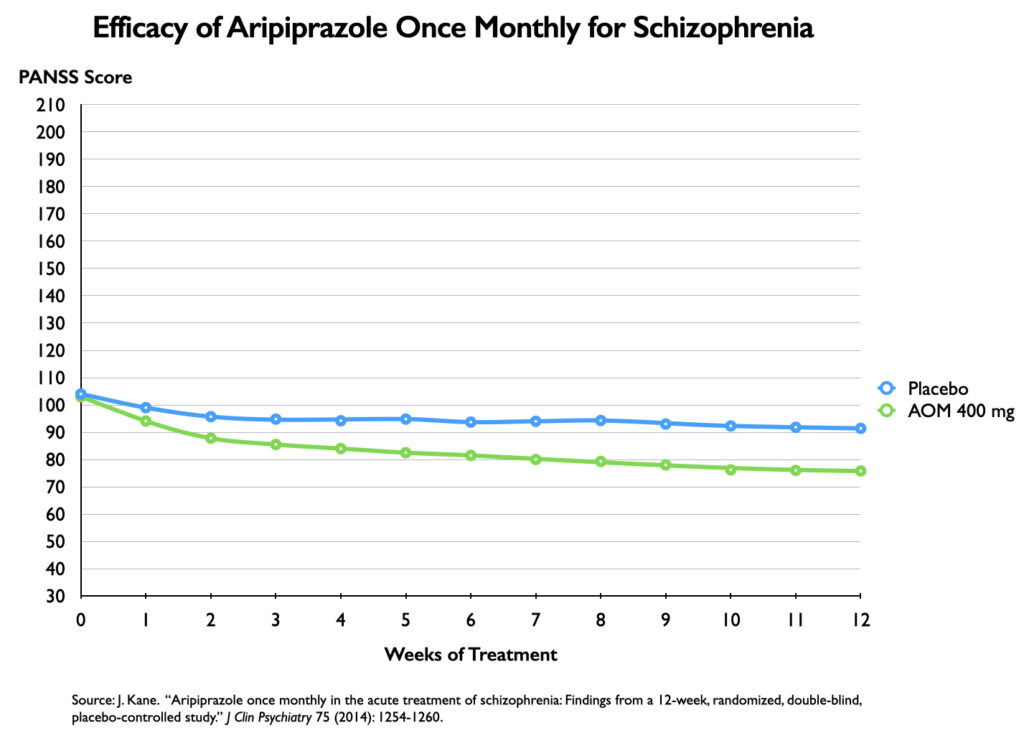
In a 12-week study of aripiprazole once monthly as a treatment for an acute exacerbation of schizophrenia, patients were randomized to placebo or to a regimen of oral aripiprazole and an injectable dose for the first two weeks, with the oral dose stopped after that period.
The efficacy graphic in the published article used a 30-point vertical axis that charted a decrease in PANSS scores over the 12 weeks (a six-fold magnification of results). The visual portrays a dramatic difference in symptom reduction between the two groups.
However, the 14.6-point difference between drug and placebo at 12 weeks still did not reach the “minimal” level of a “clinically important difference.” A graphic that charts PANSS scores on a proper vertical axis does show a visible separation in symptoms, but, at the same time, provides a context for understanding why this difference was of a modest sort and still fell short of a clinically meaningful difference.
Otsuka and Lundbeck brought Abilify Maintena to market in 2014 as a treatment for schizophrenia and as a maintenance treatment for bipolar. Medicare and Medicaid sales of the injectable from 2014 through 2019 totaled $3 billion.
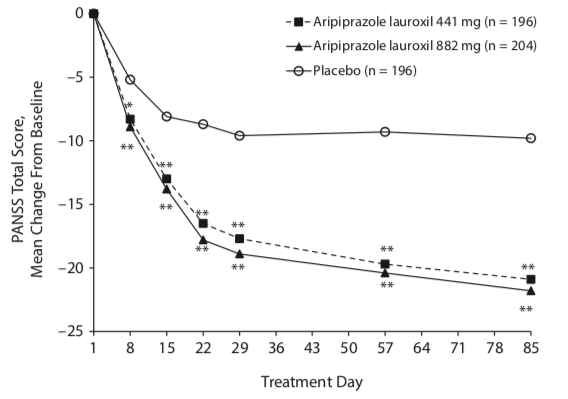
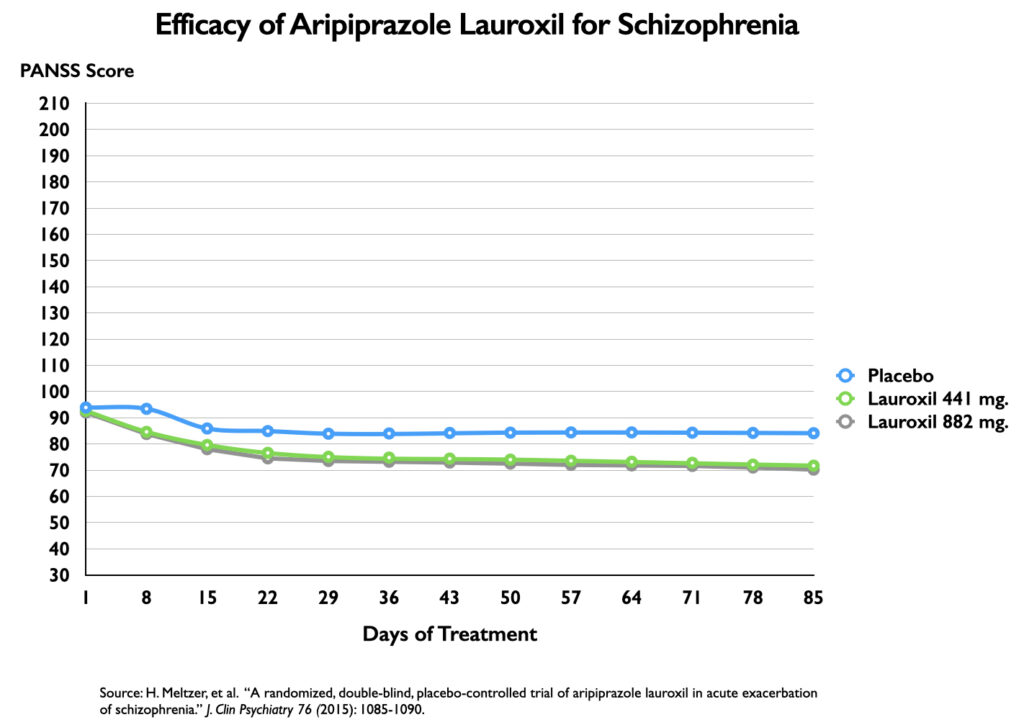
Aristada/aripiprazole lauroxil
The efficacy of the injectable aripiprazole lauroxil was assessed over a 12-week period as a treatment for an acute exacerbation of schizophrenia. The efficacy graphic in the published article used a 25-point vertical axis (a seven-fold magnification of results). The efficacy results, which compared two different doses to placebo, seem much the same as in the Abilify Maintena study.
A graphic of their PANSS scores reveals that the separation between placebo and either injectable dose is slightly less than in the Abilify Maintena trials. The drug-placebo difference appears to be of a minimal sort (12 points for the 882 mg dose, and 11 points for the 441 mg dose), and thus neither dose reached the 15-point standard for a clinically important difference.
Medicaid and Medicare sales of Aristada totaled $726 million from 2015-2019.
Drugs for tardive dyskinesia
The abnormal involuntary movement scale (AIMS) used to measure tardive dyskinesia symptoms assesses movements in seven areas, with scores of 0 to 4. Thus, it is a 28-point scale.
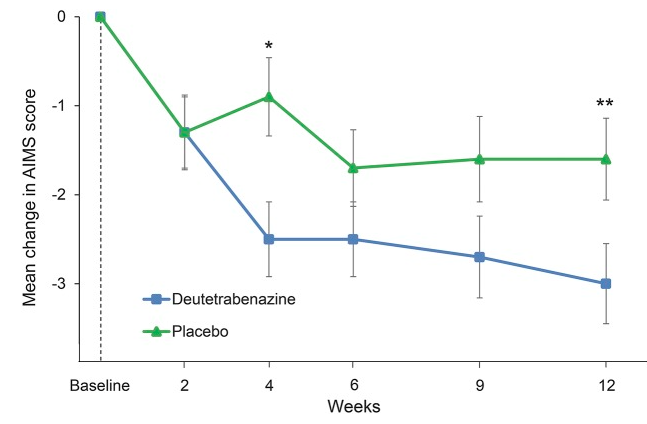
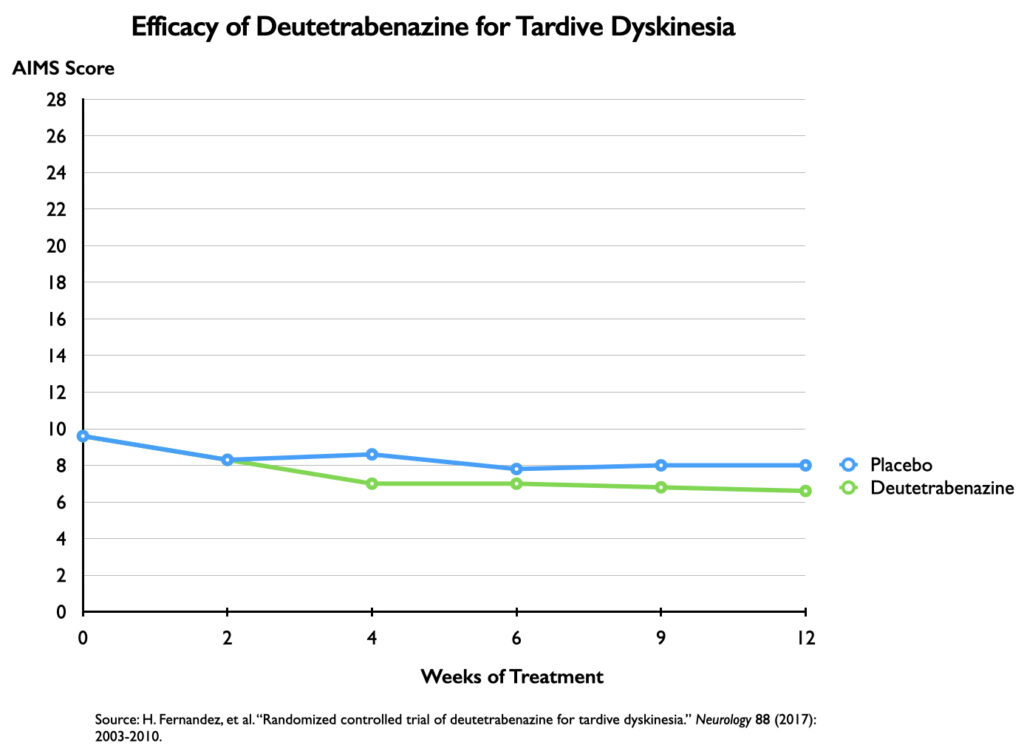
Austedo/deutetrabenazine
In a 12-week trial of deutetrabenazine, the efficacy graphic used a 3-point vertical axis for depicting drops in AIMS scores (a nine-fold magnification.) The chart depicts a dramatic decrease in TD symptoms for the deutetrabenazine group compared with placebo, with the separation evident at the end of four weeks.
In fact, at the end of 12 weeks, there was only a 1.4-point difference in reduction of symptoms on the AIMS scale between drug and placebo, with this minimal difference made clear when the symptom scores are plotted on a 28-point vertical axis.
The 1.4-point difference was deemed “statistically significant,” and thus researchers concluded that deutetrabenazine was safe and effective as a treatment for TD. However, one might think that a 1.4-point difference on a 28-point scale wouldn’t be clinically noticeable, and two secondary outcome measures proved that to be the case. There was no “statistically significant” difference in outcomes based on the clinician’s “Global Impression of Change,” and that proved to be true for the patients’ self-assessments too. Neither the clinicians nor the patients noticed a significant difference in “global outcomes” at the end of 12 weeks.
Medicaid and Medicare sales of Austedo totaled $581 million from 2017 through 2020.
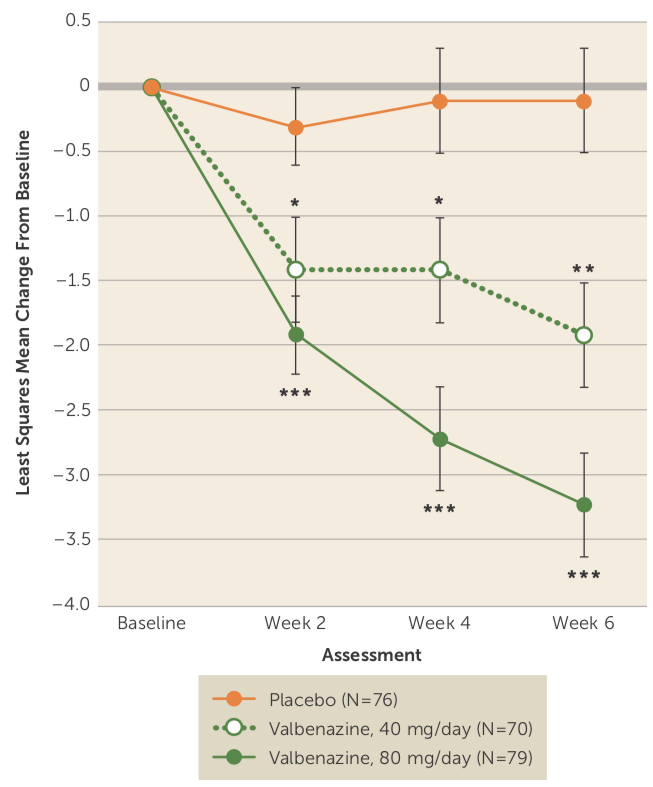
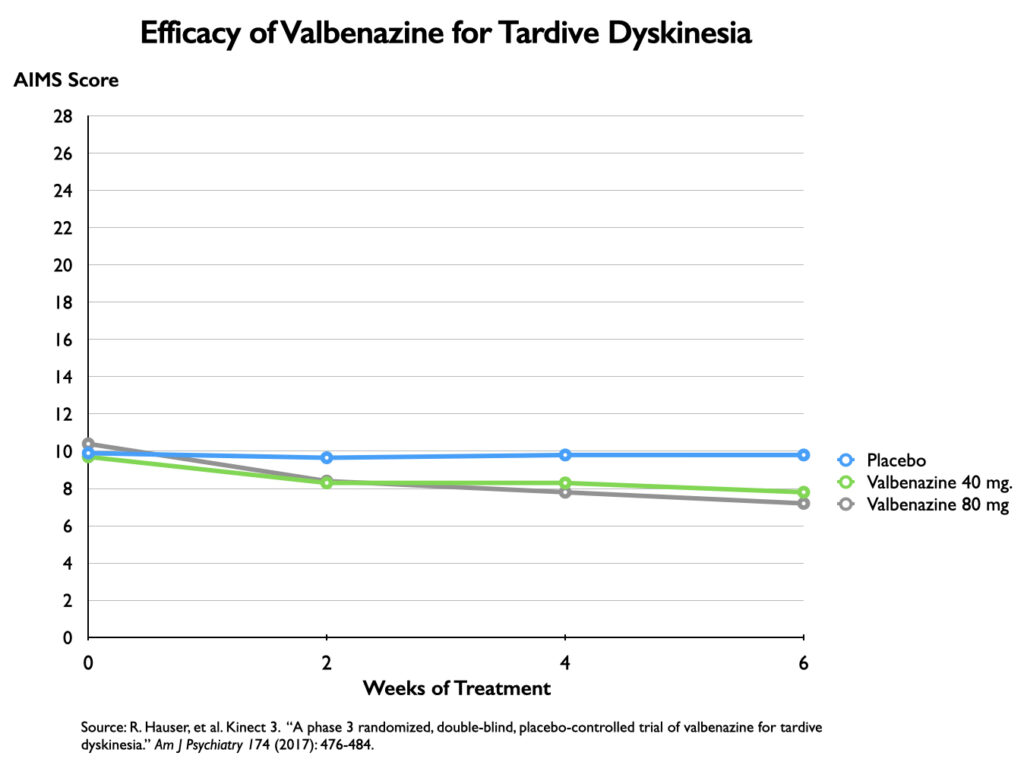
Ingrezza/valbenazine
A six-week trial of valbenazine produced a similar drop in symptoms for the medicated patients, and since the placebo patients in this study didn’t improve, the efficacy graphic that was published, using a 4-point vertical axis, showed a steep separation from placebo for both doses of valbenazine (40 mg and 80 mg).
However, charting TD symptoms on a 28-point vertical axis shows a much more modest difference between drug and placebo.
A secondary outcome measure once again reveals which of the two charts better reflects the clinical reality. At the end of six weeks, there was no “significant” difference between placebo and either drug dose on an outcome measure titled “Clinical Global Impression of Change—Tardive Dyskinesia.” This is an outcome that tells of clinicians being unable to notice a difference in the “global” outcomes of the medicated and placebo groups.
Medicare and Medicaid sales of Ingrezza totaled $1.2 billion from 2017-2019.
The Latest Antidepressant
It is now fairly well known that clinical trials of “second-generation” antidepressants failed to show much of a benefit on the HAM-D scale that was used to assess primary outcomes when these drugs came to market. In the trials of vortioxetine, an antidepressant that was approved by the FDA in 2013, the Montgomery-Asberg Depression Scale (MADRS) was used to assess efficacy. This is a 60-point scale that assesses symptoms in 10 domains, with a score of 0 to 6 for each domain.
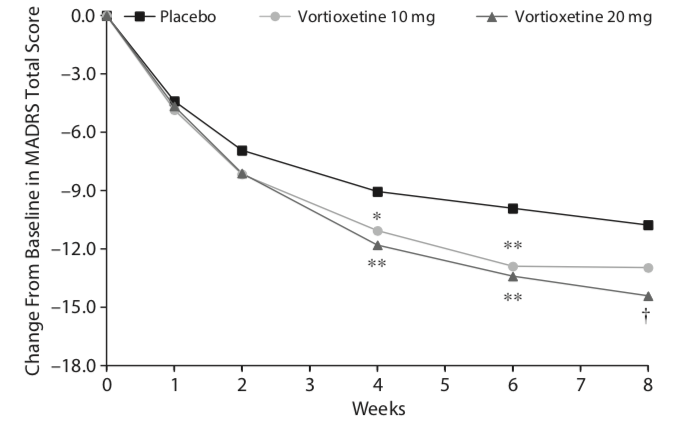
Brintellix/vortioxetine
A U.S. randomized trial of vortioxetine compared two doses of the drug with placebo over an eight-week period. The efficacy graphic in the published report used an 18-point scale to depict decreases in MADRS scores (three-fold magnification), and while it showed a separation between placebo and both drug doses, the visual separation wasn’t nearly as pronounced as in the antipsychotic trials, reflective of how the magnification effect was much less in this presentation of data.
When the MADRS scores are plotted on a 60-point axis, the difference between placebo and the two vortioxetine doses nearly disappears.
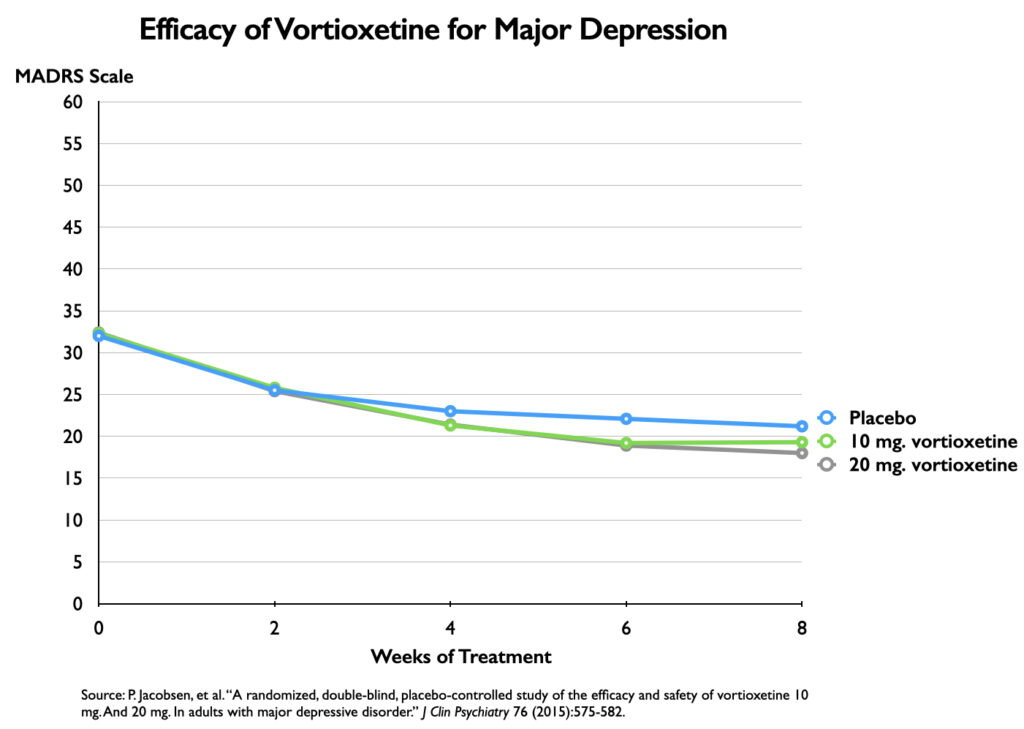 At week eight, there was a 3.2-point difference in symptoms on the MADRS scale between placebo and the 20 mg dose of vortioxetine, and a 1.9-point difference between placebo and the 10 mg dose. Although the outcomes for the two vortioxetine cohorts were nearly identical, the 20 mg dose was found to produce a benefit over placebo that was “statistically significant,” while the 10 mg dose failed to reach this standard.
At week eight, there was a 3.2-point difference in symptoms on the MADRS scale between placebo and the 20 mg dose of vortioxetine, and a 1.9-point difference between placebo and the 10 mg dose. Although the outcomes for the two vortioxetine cohorts were nearly identical, the 20 mg dose was found to produce a benefit over placebo that was “statistically significant,” while the 10 mg dose failed to reach this standard.
Other trials of vortioxetine produced similar results, with some doses deemed to provide a “statistically significant” benefit, and other doses failing to meet this standard. Even so, Takeda and Lundbeck, with the help of the psychiatrists they paid to serve as their consultants, advisors, and speakers, still found success in the marketplace with this drug, with Medicaid and Medicare sales totaling $1.25 billion from 2014 through 2019.
Visual Illusions: Key to the Marketing of Psychiatric Drugs
The published reports of the eight trials reviewed here all featured graphics that provided a “visual” of drugs that were quite effective in reducing the symptoms of the disorder. The graphics told of treatments that began providing a benefit over placebo fairly quickly, with this benefit sustained and often becoming more pronounced by the end of the study.
These efficacy graphics are what stick in the minds of prescribers. The visual presentation overrides the numerical data, and these graphics are regularly used in presentations at dinner events, conferences, and CME webinars. They tell of a separation of drug treatment from placebo over time, and thus present a visual understanding of how the clinical course of treated patients is superior to the course in untreated patients (which is how the placebo group is seen).
Yet, in the antipsychotic studies reviewed here, not a single dose of any of the five antipsychotics produced a benefit that met the standard for a “clinically important difference” over placebo. In the trials of the two TD drugs, neither drug produced a benefit over placebo that was clinically noticeable. The same was true for vortioxetine: neither drug dose produced a benefit over placebo that, on a 60-point scale, would be clinically noticeable.
In every instance, the visual picture of significant drug efficacy arose from the use of a vertical axis that served as a magnifying glass, dramatically enlarging the differences between drug and placebo.
However, as was seen above, a different picture emerges when symptoms are charted on a graphic that employs the full range of possible scores as its vertical axis. The magnifying effect is gone, and the visual is now in sync with data that tells of a drug that has failed to provide a clinically meaningful benefit.
***
Update: This article has been updated to reflect that although PANSS is a 210-point scale, the range of possible scores is 30 to 210 (a 180-point span.) The PANSS graphics previously had a y-axis of 0 to 210; the updated graphics use a y-axis of 30 to 210.



Thank you, Robert. Money distorts. You offer greater visibility.
Berta
Report comment
I give value to money. I can crave it or love it and make it my god. I don’t have to. I decide what value to place on it so that, by itself, it doesn’t distort anything.
Report comment
I have no doubt that these efficacy graphics are what stick in the minds of prescribers. But the misleading graphics are only misleading if they are not examined carefully and if other data from the studies are ignored. So the question is why are these prescribers (mostly psychiatrists I presume) so readily duped.
Some of them are probably being bought off. But what about the rest? Are they really so trusting of pharmaceutical companies that they’ll believe whatever they’re told?
Report comment
They’re so deep in they can’t bear the thought of looking at themselves and what they’ve done to get where they are.
Report comment
I wonder how small the measurable benefit has to be in order to provide relief. Placebos work. If we match the power of placebo, or exceed it even just a tad, we’ve achieved a significant result.
Report comment
The listed holy medicaments would have mummified the soft body cavities of the Pharaoh Akenaten, along with the innards of his retinue of cymbal dancers.
The blister pack has become our new canopic jars.
Not nearly as beautiful.
Report comment
Hi Bob,
https://blogs.bmj.com/bmj/2021/07/05/time-to-assume-that-health-research-is-fraudulent-until-proved-otherwise/
They’re getting away with it, because they’re being allowed to get away with it.
I believe you’ve written that people often suffer from “High Anxiety” on coming off Neuroleptics, and that its the way they deal with the “High Anxiety” that determines whether they can remain OFF the drugs or NOT.
UK Psychologists have attested that they can help people with “Schizophrenia” or “BiPolar” in the same way that they can help people that suffer from Shyness or Anxiety.
https://www.bps.org.uk/what-psychology/understanding-psychosis-and-schizophrenia
I suffered from “Catastrophic High Anxiety” when I came off Strong Neuroleptics – to such an extent that it was obvious to me that it was happening. But with investigation and good luck, I was able to figure out how to manage it. The treatment of “Schizophrenia” might COST millions of pounds, BUT recovery can cost very LITTLE.
High Anxiety can be relieved in the same way as Normal Anxiety can. DRUGS are not necessary to begin with.
Report comment
I’m sorry if I wandered off a bit, above.
Report comment
Thank you, Bob, for this informative article. It zeroes in on a very important point. I’d like to point out that these extremely unimpressive findings are in fact quite exaggerated in favor of the psychiatric drug due to problematic design and reporting features in these clinical trials such as use of inactive placebos and unblinding, placebo washout, selective publication of positive trials, etc. It’s entirely possible that with an ethical and unbiased trial design, even these tiny efficacy differences would disappear. However, differences in adverse effects would be pronounced. It’s interesting that *these* visual graphs are conspicuously missing from published trials. Drug companies and their paid psychiatric spokespersons wouldn’t want to paint that picture in prescribers’ minds, would they?
Report comment
Don’t forget the “placebo washout” strategy. They do whatever they can to reduce placebo effects and maximize the chance of a positive P value.
Report comment
As you write Brett, the industry trials are designed to favor the drug, and then there are the problems with unblinding and investigator bias (due to the unblinding and financial influences), and the problem that negative trials go unpublished. And as Steve writes, the placebo group consists of is really a drug withdrawal group. What is astonishing is that in spite of all these biases that favor the study drug, the published trials still show only a minimal benefit. This suggests that if you ran trials that were free of industry influence and investigator bias, and did so in medication naive patients, then the efficacy benefit would not only disappear, but rather that placebo might produce superior outcomes in terms of reduction of symptoms.
Report comment
to insist that problems with thought, emotions, drive, impulse control, cognition, perception, behaviour and so forth should be exempted from the rubric of illness, and the assertion that there is no such thing at all as psychopathology, seem entirely unjustified.
absolutely true
Report comment
Sure, those problems are devastating. And most people would be desperate for help when they are in any sort of pain. And?
Report comment
I shouldn’t say “are devastating”, but rather they “can be devastating” depending on the individual and his/her circumstances.
Suffering is real. Depression, anxiety, panic, inattention etc. and the whole gamut of problems in thinking, living and feeling human beings have are real. I think everyone, even here, acknowledges that.
Report comment
The answer is included
Report comment
So lets opt for a cult called psychiatry. There is nothing “medical” about these people.
Report comment
A pretty simple solution to find out what these drugs do is to just take them. Clearly taking a single SSRI or neuroleptic will not kill you. It should be required for anyone who wants to prescribe them.
I can not speak for neuroleptics, but the first pill of SSRI I ever took was an outlandish experience. I’ve never taken any of the classic upper street drugs, but I imagine them to be similar. I’ve never felt as strong in my life. Followed within a few days by the ralization that this was nothing but an illusion created by a drug, so I quit them right away.
I can understand people getting addicted to them with basically the first pill.
Report comment
What is the standard deviation for any of the data points?
Report comment
In the article, there are links to the published articles, which provide the standard deviation data for the outcomes for the different groups.
Report comment
“In any case, lots of good medications become ineffective eventually,” adds Michael Milham, MD, PhD…that doesn’t mean we don’t use them for as long as they work.”
“The largest of the controlled studies, called the MTA (or Multi-Modal Treatment Study of ADHD) study, treated nearly 600 children in the late 1990s for 14 months. The longest treated over 100 kids for 2 years. Both found that kids treated with stimulant medications had their symptoms significantly reduced, and the effect was more powerful than in kids treated with behavioral therapy.”
Report comment
Yet in the 3-, 5- and 8-year follow-ups on this very same study, kids who continued or started to use stimulants did no better (and in some ways worse) than kids who discontinued or never used stimulants. Which fits in exactly with the data I just shared in another post, and which is generally well known but kept very quiet among researchers: stimulants do work to “control the core symptoms of ADHD,” as they usually put it, but have been shown again and again not to lead to any improvement in long-term outcomes. In other words, stimulants can make it easier for you to pay attention to your schoolwork and complete your homework, but apparently, paying attention to your schoolwork and completing your homework doesn’t lead to you becoming more successful later in life.
In fairness, there are many who claim that stimulants destroy kids’ lives, and while that may be true in individual cases, in the collective, there is no evidence of stimulants doing great HARM to long-term outcomes in the collective. But they don’t really help, either, if you care about things like delinquency rates or high school graduation rates or social skills. And there are risks, outlined in my other post, that a rational parent might be well advised to consider when that risk is set against only short-term gains. It is a matter of informed consent, and it’s pretty dishonest at this point to claim that stimulants are going to magically change kids’ lives for the better in the long run. Some may choose to use stimulants for their short-term ability to “reduce the core symptoms of the disorder,” but if they’re aiming for longer-term improvements, it appears they have to look for additional interventions to help.
Report comment
Some honesty in advertising would be welcome here:
“This pill might make you 5% less depressed/schizophrenic, but you will become lethargic, fat, asexual and drug addicted.”
Great deal.
Report comment
“A picture paints a thousand words,” and that goes for big Pharma’s fraud, and the artists’ work. It’s a shame the psychiatric and psychological industries seemingly forgot this slice of wisdom.
Thanks for pointing out the power of pictures, Robert – whether they’re utilized to deceive – or are “too truthful” renderings of the psychiatrists’ and psychologists’ blunders.
Report comment
Not “a systematic, scientifically controlled way”
Research on long-term effectiveness
There are a number of studies that follow children for longer periods, even into adulthood, but the kids in these studies are not being treated in
{a systematic, scientifically controlled way}
so the results are not conclusive.
Bob, would you consider writing up a thorough review of the breakthrough news that, “CDH2 mutation affecting N-cadherin function causes attention-deficit hyperactivity disorder in humans and mice”
Thanks
This is a star studded group of experts extraordinaire: D. Halperin, A. Stavsky, R. Kadir, M. Drabkin, O. Wormser, Y. Yogev, V. Dolgin, R. Proskorovski-Ohayon, Y. Perez, H. Nudelman, O. Stoler, B. Rotblat, T. Lifschytz, A. Lotan, G. Meiri, D. Gitler & O. S. Birk
Report comment
N=3 ….
It’s trash masquerading as science.
Report comment
What about congressional hearings? Mad in America does so much work, but what about getting it front of elected officials and the mainstream press? Is there any senator or congressperson open to battling Big Pharma and the status quo of Big Psychiatry? Is there any hope on the horizon?
Report comment
How do you live and fight on without any reasonable sane hope that torture from criminal (not forensic) psychiatry will end?
How do I protect myself as I know I am being torn to pieces (it’s been almost a decade, now 58 years old)?
I can’t stop the pain applied from without and its destruction, though I’ve tried every day, so what I am supposed to do next every day when nothing works and there is no hope and I know I am being worn down, as I being worn down, on unemployment and medical welfare, losing my ability to walk and feeling the torture more than ever in my mind?
How do you move forward alone alone alone with no answers to these questions?
How do I survive criminal psychiatric impoverishment and total mind, body, wallet, social annihilation?
I can’t risk and do not believe in psychiatry or psychology, or religion or family or government, who have not been on my side.
Thank you for the space to shout into the void as I sink/get pushed down/can’t fight back right enough to win.
https://ginafournierauthor.com/
Report comment
edit (I need more time): “as I am being worn down”
(and a delete button, if not justice)
What do I do? I testify pretty clear of the risks.
I don’t think it wise, may change my mind, to try and claim disability caused by suicide swatting and illegal looney bin lock up, not acknowledged, causing mental torture. Too convoluted.
I have been made unbelievable, by my actual story. That’s mental torture.
I took the drugs (list available) only when forced, while locked up, due to premediated action and suicide swatting. I was not suicidal, I need to rebutt. I said “save my life” on social media, the opposite.
I took the drugs after I heard from other locked up patients about promised release denied by medication levels too low, apparent in blood testing. I did not know about blood testing for Big Pharma drug levels.
Got records. Before and after they were purged and slightly altered by hinterlands Catholic hospital. I did not swallow the drugs at first. I took the drugs only about three or four days when I worried I needed to in order to get out. That’s mental abuse, not aid.
And when I was released and I ran out, after a week, but still forever and ever-more branded, I did not fill the prescriptions, paper copies which I retain, from about February 27, 2013.
No one needed to tell me the drugs were bust. That fact has not helped me. I am screwed up like I was gaslit (I was/am gaslit) and I took the drugs to screw me up further, medicating normal and messed up.
I should be studied for psychiatric damaged caused by psychiatry, like a child raised by wolves. Anything to safely put my story on the right track.
There is no safe rhetoric for my story. Not for me. My mountains of documentation need explication. I got no one else to defend me but me, which is not allowed.
I need help out of this hell, safe help.
Report comment
Gina: you are believed.
Report comment
N-Cadherin belongs to a superfamily of calcium-dependent transmembrane adhesion proteins. It mediates adhesion in the intercalated discs at the termini of cardiomyocytes thereby serving as anchor for myofibrils at cell-cell contacts.
Just the briefest of definitions regarding N-Cadherin
Report comment
Using selected nutrients instead of psych drugs and having done so for years, I find these arguments fairly bizarre, particularly since there’s no real improvement in the treatment groups, although there is a great deal of company puffery about their newest drugs after the alleged studies are done.
Report comment
“More recently, however, experts have concluded that reacting to a placebo is not proof that a certain treatment doesn’t work, but rather that another, non-pharmacological mechanism may be present.”
“The power of the placebo effect”
Harvard Health
Aug 9, 2019
Bob, how do you account for the hundreds of millions of people whose lives have been spared through the drugs produced by BP? Certainly, not every one of them was helped through the placebo effect. If they weren’t having a considerable impact, coming off them wouldn’t be such a nightmare. If the drug trials are fixed in favor of the drug company, why do they spend millions developing them? Couldn’t they throw some innocuous chemicals together and achieve the same results?
When psychiatrists first found that chemicals could relieve some of the pain of depression, they were surprised. Didn’t they soon realize they were on to something potentially groundbraking?
Report comment
Removed for moderation.
Report comment
Enrico, I sometimes like your divergence of inquiry, it freshens the exploration, but I have to say the drugs ARE such a nightmare to come off for many other reasons. My friend Elaine phoned me from a hospital bed this afternoon. She was antipsychotic free for three months, on her choice, and she needed support to continue that process, but she only had her medical team parroting that she was a bad girl for quitting and so they would not give her ANY support other than the drugs she hates. So instead they put her back on a very high dose of antipsychotics. She is now in the general emergency hospital from going unconscious on a train. I imagine that her gradually improving and stabilizing brain and body, over three months, did not know how to function when she was thumped back on a high dose. In this way I suspect the body shock of going from withdrawal to chemical overload is perilous, if not negligant. Much like taking an ex alcohol dependant withdrawer and pickling them in a vat of vodka.
I am not anti pills per se. The whole planet is awash with forever chemicals so it is not like any human has an unpolluted body and brain. Even placentas are turning attractive shades of micro plastic. Im not a pill nun. I do think in a crisis pills can rarely but certainly have a function. Some can be convenient as quasi placebos, a nice xmas cracker of capsules to shake, a gift to give someone balancing on the microwave antenna of a tall building, like a box to rattle rather than their bones ending up rattling in a box.
I have done the pill circuit. It kept me alive certainly so…..but only by one thing. Hope. And that is not to be sneezed at when psychosis makes hope vanishingly small. But longterm the damage seems to me inexcusable. There are other forms of hope. Non pharma chemical forms. More holistic forms. These should be tried first. But these are only my own personal opinions and I have no desire to waste my day backing them up with scientific rigour.
I think it is possible to scrape out a persons brain with chemicals such that they go around brain free and whistling and “feelin good”. When the brain is put back again it is inevitable this will feel like a nightmare initially. It is not always simply that the inner panic skirmish from a lack of chemicals means the chemicals were needed. Any more than alcohol is needed. And as with hope, there are undoubtedly safer ways to go brain free for a while than toxins.
Sex, for instance.
A post coital cuddle has chemicals. Ocytocin et al.
Report comment
Since, a person who posted misspelled “groundbraking” instead of “groundbreaking” it can only be concluded they are really “groundbraking.” As that these drugs do actually act like “brakes” on people’s lives. They are “brakes” in that while the person is taking these drugs, their lives stop—they become what many of us fear the most—“zombies.” Outside the person appears alive, inside the person is dead, dead, dead — “dead as a doorknob.” When someone comes off the drugs, they spring to life, however, they still must go through a withdrawal period and then afterwards must adapt to a “post drug life” where they must consider they now have some brain damage and perhaps some damage or at least confusion in other bodily systems. Of course, what is damaged, etc. in either the body or brain is highly individual. The main thing is that coming off the drugs gives a person the will to live. Staying on the drugs cause someone to essential be dead. So, yes these drugs are “groundbraking” as they brake people into being useless nothings. But there is hope! Get off the drugs! Walk away from psychiatry! And dare I say, Trust Jesus! Thank you.
Report comment
“Published reports of clinical trials of psychiatric drugs typically include a graphic showing the efficacy of the study drug in reducing symptoms of the disorder compared with placebo. These graphics are visually compelling,” – Robert Whitaker. Too true Bob, yet is it any wonder in a human world driven by optical delusions of consciousness?
As Albert Einstein concluded: “A human being is a part of the whole called by us universe, a part limited in time and space. He experiences himself, his thoughts and feeling as something separated from the rest, a kind of optical delusion of his consciousness. This delusion is a kind of prison for us, restricting us to our personal desires and to affection for a few persons nearest to us. Our task must be to free ourselves from this prison by widening our circle of compassion to embrace all living creatures and the whole of nature in its beauty.”
I just bought Iain McGilchrist’s The Matter With Things: Our Brains, Our Delusions and the Unmaking of the World. Interested to see what he brings to Einstein’s “no problem can be solved from the same level of consciousness that created it?” Iain writes:
“At one point I was going to call this book There Are No Things. I changed my mind when I saw that it might align me with a nihilistic trend in post-modernism that I deplore. It also gave the impression that I was arguing for ‘truth-as-correctness’ rather than ‘truth-as-unconcealing’.” He offers the book as a hopeful synthesis of science & philosophy.
In the meantime the politics of experience continues unabated, I see.
Report comment
So lets opt for a cult called psychiatry. There is nothing “medical” about these people.
Report comment
Thanks Robert.
Psychiatry won’t change so hopefully enough people become educated enough, questioning enough to stay the hell away from not just psych but also GP’s that are indoctrinated to see psych as a convenient “tool”.
Report comment