Two years ago, I wrote an article for MIA about an experimental clinical trial called The Broaden Trial. It was, and perhaps still remains, the largest clinical trial to investigate the efficacy of deep brain stimulation for depression. The trial had been terminated in 2013, due to a low 17% success rate among at least 75 patients that received the controversial brain implant. But the public was given no additional details from the sponsor about the trial’s protocols, or the outcome of at least 128 people that had enrolled in this risky experimental study.
In October, 2017, I received an email from Jim,* one of the three trial participants I interviewed in 2015. “Finally, they published a report!” Jim wrote.
The Broaden Trial results had been published in that month’s Lancet. It was a relief to Jim and the other trial participants I had interviewed. All had suffered a range of debilitating adverse effects, and ultimately had the device explanted. And they had spent years worrying about the fate of the other trial participants, as DBS experimental trials continued at a rapid pace—for depression, OCD, PTSD, schizophrenia, addiction, and a growing list of psychiatric disorders and neurological diseases.
The Lancet paper provided some answers to the many questions surrounding this study, but a number of those “answers” were in stark contrast to the reports of trial participants. It also left many questions unanswered—about the integrity of the clinical trial process, and the efficacy, safety, feasibility and ethical concerns related to brain implants, which have the capacity to dramatically alter mood, personality, cognition and our definition of what it means to be human.
Trial Background
The Broaden Trial began in 2008. It was the first trial of its kind: a large, multi-center, randomised double-blind trial of DBS for treatment-resistant depression. The trial was sponsored by St. Jude Medical (SJM), a medical device manufacturer, and a big player in the field of cardiac pacemakers. SJM was eager to diversify and become a top contender among a handful of companies selling DBS brain implants, including Medtronic, the first company to get FDA approval for essential tremor in 1997, and for Parkinson’s in 2002.
At that time, the so-called “neuromodulation” market was estimated to double in growth, to $8.8 billion, by 2012. In 2005, SJM invested $1.3 billion to purchase Advanced Neuromodulation Systems, a company that specialized in neurostimulation implants, which were already being studied in a number of DBS trials, including a Canadian study of DBS for depression. A few other device companies were also sponsoring small DBS trials for depression, OCD and Tourette’s Syndrome, often with subsequent, highly touted success rates, though the brain target for the implants varied from one disorder to the next. Even among depression trials, different researchers preferred different brain targets, and at least five different brain targets were used. And these small initial studies were typically open trials, which was why the Broaden Trial was such a landmark study for DBS.
When the Broaden Trial kicked off, SJM had not received FDA approval of its DBS device to treat any condition. The company was given FDA approval to conduct the study under an investigational device exemption, a process that has been criticized because it allows medical device companies to conduct studies more quickly and for less money because they can skip the typical Phase I and Phase II trials required for pharmaceutical companies.
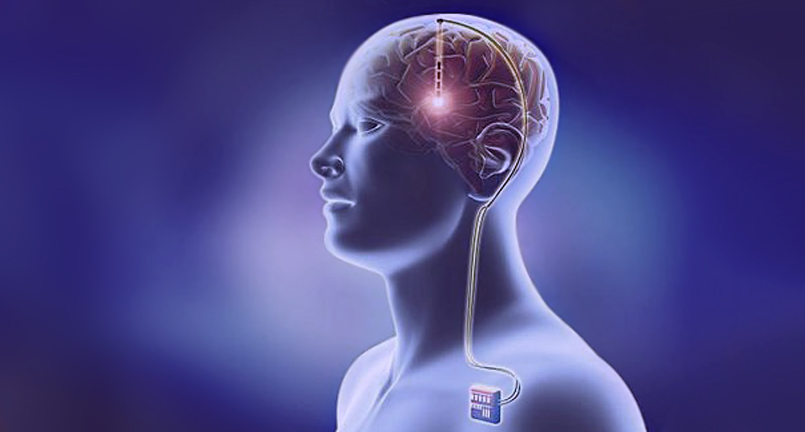
This FDA fast-tracking is especially controversial given the invasive nature of DBS, which requires chest and brain surgeries, and allows for chronic brain stimulation that can lead to dramatic mood and personality changes. In the Broaden Trial, two sets of brain stimulators were implanted into a region of the cerebral cortex called the subcallosal cingulate gyrus. The DBS implant is nicknamed a “brain pacemaker” because it’s connected by wires to a computerized device implanted in the chest, via wires that tunnel through the side of the neck. This DBS system is operated by remote control, by an external software program set to shock the brain constantly, at a voltage ranging from 0.1 to 10 volts.
The Broaden trial ultimately signed up 201 patients. But the FDA required a futility analysis of “at least 75” of the initial implanted patients at the six-month mark. The Lancet paper revealed that 90 patients were implanted between 2008 and 2013, and their data analysis revealed an estimated success rate of only 17%—much lower than the 78% response rate initially advertised in June 2008, soon after the Broaden trial began and SJM began to solicit patients. In late 2013, SJM decided to terminate the trial, even though the results were still slightly higher than the FDA’s definition of futility: a 10% success rate. By that point, the trial had been going on for almost five years, collecting follow-up data that is detailed below.
Despite the disappointing results of the Broaden trial, at the start of this year, Abbott Pharmaceuticals bought SJM for $23.6 billion. SJM had received FDA approval of its Libra neurostimulation implant in 2015, but only for the movement disorders. According to Abbott’s press release, the purchase “creates one of the broadest medical device portfolios in the world and provides a steady stream of new technologies and therapies,” including “attractive markets,” “significant and sustainable value creation for shareholders” and “a powerful pipeline across cardiovascular and neuromodulation patient care ready to deliver next-generation medical technologies.”
According to Abbott’s third quarter earnings report, their 2017 medical device sales in the first nine months of the year were $2.6 billion. The neuromodulation segment generated $590 million in revenues during this period. That’s up from their first nine-month revenue in 2016, which was $397 million for the SJM neuromodulation devices.
Conflicting Interests
Critics and watchdogs of DBS research, including some DBS proponents, have said that the DBS research field is uniquely vulnerable to conflicts of interest, researcher bias and lack of transparency. Until the Broaden trial paper was published, the public, including trial participants, had access to a tiny bit of information about the trial on ClinicalTrials.gov, either by being privy to the trial’s registration number, or by hunting through the many DBS studies and making a guess. But the only information to find is a brief statement that the “trial of that device is not approved or cleared by the US FDA.” The page doesn’t mention a sponsor or medical provider. The “Responsible Party” is listed as “Redacted.”
The report of the Broaden Trial published in Lancet is authored by a list of 30 contributors from 13 medical centers. The majority of these centers are established university-affiliated hospitals, including Stanford, UCLA and NYU. Seventeen of the 30 authors have received grant money and/or consulting fees from SJM; one author was an employee of Abbott at that time, and two Abbott employees provided statistical analysis.
Many of the authors had also received fees from two other major DBS manufacturers: Medtronic (10 doctors) and Boston Scientific (4 doctors). Two of the doctors on the list of authors, neurologist Helen Mayberg and neurosurgeon Dr. Andres Lozano, had also licensed intellectual property to SJM, and had been responsible for the initial proof of principle study of DBS of the subcallosal cingulate gyrus for depression.
Participating in a trial is time-consuming, for both citizens and medical providers—particularly a trial involving brain surgery. But the investigators in the trials aren’t required to divulge how much financial support they receive from device manufacturers for their participation. This is a very expensive intervention. In a non-trial setting, medical providers charge high fees for their services and the DBS device is also pricey. Here’s a general overview of the costs:
- The DBS brain and chest surgeries cost between $65,000 and $100,000, including the implants, which make up approximately $20,000 of the sticker price.
- Surgery to replace the chest pacemaker is approximately $14,000, and replacement is necessary every 5 to 7 years with the new rechargeable battery packs (compared to the old battery packs that had to be replaced within one to two years, or sooner).
- There are also the additional costs of follow-up care, such as the necessary programming sessions (to have the brain implant device settings modified), other psychiatric follow-up sessions, and often additional brain surgeries, to fix or replace broken hardware, or to have the devices explanted.
Broaden trial recipients got the related medical care for free. Jim, Rich,** Steve Ogburn, and perhaps many other trial participants, were motivated to participate in the trial because this was the only way to get access to this treatment without having to pay for it themselves, as insurance won’t cover experimental treatments. However, when they volunteered for the trial, Jim, Rich, Steve and undoubtedly many others didn’t know that many of the investigators had their own financial incentives, particularly those receiving licensing fees for innovations in DBS treatments.
Deconstructing the Trial Results
Missing info: What device was implanted?
In 2008, the Broaden trial was launched with a catchy moniker, a nod to both the size of the trial and the target region of the brain implant, which is known as Brodmann Area 25. The campaign launched with much hype, on the heels of some very promising early research from a smaller Canadian trial. This initial hype received a lot of positive media attention, both because it was hailed as a promising money-maker for SJM and its investors, and as a novel therapy for severe depression. As a result, more than 1,000 people with depression sought to participate in a DBS trial.
On CNN, Dr. Sanjay Gupta discusses the “astonishing” results from the initial trial.
Given the initial hope and hype about the trial, it’s noteworthy that the Lancet paper never once mentions the moniker “Broaden.” The paper only includes the trial’s registration number, which identifies it as the Broaden study. The paper also doesn’t mention the name of SJM’s Libra DBS device, which was the company’s only available DBS device at that time. This was the model used in every past SJM-sponsored clinical trial, and is the model that gained FDA approval in 2015 as a treatment for Parkinson’s and essential tremor.
I recently went shopping for the Libra implant device on St. Jude’s website, but nothing came up. The company has since developed a newer model, called Infinity. Infinity received FDA clearance for two disorders, Parkinson’s and essential tremor (movement disorders), in October, 2016. Its features include “upgradeable technology,” allowing “access to approved future innovations without surgery through app-based software updates.” Another new feature is a sleek Apple wireless control device that lets users change the implant’s settings, primarily turning the stimulation up or down. According to an SJM media rep, it also has “improved durability and enhanced comfort” and “directional leads” that allows programmers to “avoid areas that could cause side effects.” SJM has released a PR video of the new device.
For the many patients involved in the pre-market trials that led to FDA approval, upgrading to the new implant would be costly. It would require two additional complicated, risky and expensive neurosurgeries—to explant the old Libra device and to implant the new device. In other words, the Broaden trial, and all the trials meant to establish the safety and efficacy of St. Jude’s DBS implant, involved a product that is already out-of-date, and no longer promoted.
Efficacy Rates: Double-blind, open label, and patient accounts
The Broaden trial was blinded and sham-controlled for the first six months. Two weeks after the chest and brain surgery, 60 people began receiving stimulation and 30 were in the sham group (a surgical equivalent to placebo control), but recipients and researchers did not know whether patients were receiving the active treatment or the sham treatment.
During the initial six months, five patients exited the study, including one person in the control group; three were due to adverse effects. At the six-month checkpoint, among the 85 remaining patients, 20% of the stimulated patients and 17% of people that received the sham treatment were said to benefit from the treatment. The overwhelming majority of volunteers in the trial didn’t benefit during this double-blind phase of the trial, regardless of which group they were in (active or sham treatment).
After the initial six-month blinded period, the trial turned into an open-label study, meaning that the 30 people receiving sham DBS began receiving stimulation, and the participants and investigators were no longer “blinded.” When this happens, physicians are often prone to becoming less objective and interpreting results and outcomes as positive.
During the next six months, another five people left the study, including three due to adverse events. At 12 months, 28.5% of the 80 participants still in the study were classified as responders and 12.5% were in remittance, which was a slight improvement over the six-month outcomes. (Response rates were based on a 40% or more reduction in MADRS scores; remittance meant a MADRS score of 10 or lower.)
These increased response rates over the six-to-12-month period, which are often seen when a blinded trial becomes open label, nevertheless contrast with earlier non-blinded “open” trials of DBS of the subcallosal cingulate gyrus, which found that response rates decreased over time. And in this Broaden trial, efficacy rates continued to increase past the one-year mark, and by the end of 30 months, with 79 patients still in the trial, 46% were considered responders, and 19% were in remission.
The published report tells of a therapy that, at the end of two years, had helped two-thirds of those who stayed in the study. But this efficacy only showed up in the open-label part of the trial, and not during the placebo-controlled phase, when the investigators were “blinded” to who was getting the treatment.
Jim was classified as a “responder” during the 24 months he was in the trial. I had interviewed him for my earlier article for MIA. A lawyer from California, he had to go on disability after he received the implant, and he was shocked to discover, after he received his medical records in 2015, that he had been classified as a responder.
“All the way along, I reported to my doctor, ‘I’m not getting any benefits,'” he said in 2015. Post-op, he had a lot of cognitive issues, and during the double-blind phase he thought he was in the “off” group. But at six months, when everyone started receiving stimulation, he began to have a “profound feeling of disconnection with reality,” anhedonia and a range of adverse effects (documented in my 2015 article for MIA). After approximately two years, Jim had the device turned off , and he said his “thinking started clearing up a bit.” But the explant, which was four hours long and very painful, caused a new range of post-operative cognitive issues, and the psychic ache of depression also increased.
Stimulation-related adverse events
According to the Lancet paper, the “optimal” brain target was chosen by “at least two of three experts” (neuropsychiatrist Paul Holtzheimer, neurologist Helen Mayberg and neurosurgeon Chris Honey). It was “defined as a region in the subcallosal cingulate white matter approximately 75% of the distance from the anterior commissure to the plane defined by the grey matter edge of the genu of the corpus callosum and in the transition from the white matter to the grey matter in the medial–lateral axis.”
Neurosurgeons have been targeting this brain region since the 1960s, with a psychiatric neurosurgery called “cingulotomy.” More recently, it was linked to sadness by research conducted by Helen Mayberg, one of the innovators and patent-holders of DBS of the cingulate gyrus. It is also known that the cerebral cortex is a densely packed region, and that the targeted region is flush with serotonin-reuptake transporters; that it controls executive and cognitive functions; is linked to a range of emotions (fear, anxiety, reward, memory recall); and is connected to a vast network of brain regions including the hypothalamus, the amygdala and the hippocampus.
In 2015, I spoke with the Chicago-based Broaden trial neurosurgeon who said that the size and location of the subcallosal cingulate varies from one person to the next, and is thought to be approximately 2-3 mm in size. The Lancet paper revealed that four trial participants required a second surgery, after post-op scans revealed that the implant wasn’t “in the ideal target region.”
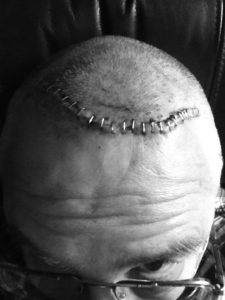
Each of the two (bilateral) brain implants has four electrodes sized 1.5mm, and there are two sizes available, with space of either 0.5 or 1.5 mm between each electrode. The paper doesn’t mention which size implant was used, which is important given the size variable of 2 to 6 mm. The surgery and the implant are known to cause microlesions in the brain, and the right hemisphere is typically larger than the left hemisphere, so lesions and also stimulation effects can also vary dramatically from one millimeter to the next, and from one side of the brain to the other.
The DBS implant can be programmed for a wide variety of stimulation settings. At the start of the Broaden trial, only one of the four electrodes on each of the two (bilateral) brain implants was turned on for stimulation. The initial amplitude of the stimulation was 4 milliamps, at 130 hertz, 91 microsecond pulse width. Two weeks later, if recipients didn’t have a 10% reduction in their MADRS scores, the voltage was increased to 6 milliamps. After an additional four weeks, if there was no benefit, voltage was increased again to 8 milliamps.
At the fourth check-up (10 weeks after initial programming), if there was still no benefit, and no “intolerable side-effects,” a second electrode beside the ideal target electrode would be turned on at 4 milliamps, and the first electrode would also be reset to 4 milliamps. If the patient had no immediate negative effects, that setting would remain throughout the rest of the trial because no further setting changes were allowed after the 10-week stage.
This protocol, and higher voltages in general, are known to expose patients to greater risks, particularly adverse mood and personality changes like mania. DBS research has shown that even 1mA of electricity affects brain tissue 40 mm3 away, and at 3 mA, the range is up to 140 mm3. Even one contact stimulating at 1 mA stimulates a large area, and this in a region known as the CEO of the brain, controlling a vast range of emotions and behaviors.
However, the authors of the Lancet study provide no details about the actual voltage settings of participants. Instead, they reported that “no side-effects occurred with DBS programming, and no parameter adjustments were required due to side-effects after a parameter change.”
This statement is belied by the personal reports of Jim, Rich, and two other Broaden trial participants I interviewed. All told of stimulation-related adverse effects that necessitated adjustments.
Rich had extreme and deeply disturbing effects from stimulation throughout his three-year participation in the trial, particularly mania and impulse control. In my 2015 article, Rich chronicled a range of horrifying stimulation-related effects that he described as occurring suddenly, as if he were on auto-pilot. Rich said that he reported every detail to the trial center. After he attacked himself with a knife, he turned the implant off himself, which patients can do by holding a magnetic wand over the chest pacemaker. He said his doctors wanted to turn it back on, and a year later, he finally agreed to that, as long at the programmer in charge of his brain implant agreed to a maximum of one volt. But he continued to experience a range of behavioral issues, and had many cognitive and physiological issues even after the device was explanted.
Another trial participant, a woman who received the implant in 2012, at the age of 30, told me that she was hospitalized twice after her device was turned on. She did say, though, when I first interviewed her, that she ultimately benefited from DBS and considers it successful, despite her initial adverse reaction to the implant. However, since we first emailed in 2015, she hasn’t responded to my emails.
The fifth participant I spoke with said that while the DBS has helped curb his depression, this relief came only when his programmer went off-label from the stimulation protocol. He also suffered many stimulation-related adverse effects, including panic attacks, anxiety and sudden anger. “A lot of us have had anxiety or anger come on for no particular reason,” he said, when I spoke with him in 2016. Yet, his trial psychiatrist classified him as a remitter, meaning that he had—in terms of the reported trial results—the best possible outcome in terms of efficacy.
In sum, while the published paper told of no side effects related to the programming of the implant, all of the patients I interviewed told of programming-related adverse effects.
Serious adverse events
Although the Broaden investigators said there were no side effects associated with DBS programming, they did publish a long list of general “serious” adverse events that the trial participants experienced in the first year and also during the follow-up, which was scheduled to continue for five years.
In the first year, the Broaden authors reported that 28 people had 40 serious adverse effects. The authors stated that “Eight serious adverse events occurring in seven patients were judged to be definitely related to the study device or surgery, including six infections (in five patients), one skin erosion over the extension wires, and one postoperative seizure.”
The other 32 serious adverse events were chalked up to the disease, and not to the treatment.
Two people in the study died by suicide. Both were in the control group, but they committed suicide after they started receiving stimulation. Eight other “early” study exits included “one worsening depression, one suicide attempt, one increased suicidal ideation with failed rescue, and one head pain.” There were a total of five suicide attempts, and two other people experienced suicidal ideation. Seven people were hospitalized, four in the second year of the trial.
However, the Broaden authors write, in their Lancet paper, that these “serious adverse events were attributed to the primary mood disorder and deemed unrelated to the device or active stimulation.”
Yet, there is other research, involving studies of Parkinson’s patients, that have found a link between DBS and higher suicidal behaviors. In one study, suicide was higher than the highest of WHO’s matched population rates. Another study found that suicide rates for Parkinson’s patients treated with DBS were twice as high as rates for Parkinson’s patients treated with conventional drug therapy.
One Broaden trial participant who became suicidal was interviewed by neuroethicist Frederic Gilbert, who published a case report in Neuroethics. (Gilbert also interviewed Rich and published a paper about him.) The 32-year-old woman reported post-op “depersonalization,” and a sense of powerlessness, saying, “It felt as I was living on ‘auto-pilot.'” Gilbert notes that “postoperative feelings described by the above patient are not rare. A significant number of patients have reported experiencing a postoperative sense of self-estrangement.” The woman told Gilbert that the “unexpected worsening of these feelings played a very significant role” in her increased suicidality. Less than three months into the trial, she was hospitalized. Ten months into the trial, she went to a rooftop, but luckily the police got to her in time and she was re-hospitalized. At month 14, after a second suicide attempt, the trial doctor turned off the brain stimulator.
This detailed description of very serious, troubling, continued post-implant effects, by a knowledgeable neuroethicist, certainly sounds attributable to the treatment. Yet, by choosing to attribute it and other suicide attempts and mood-related hospitalizations to the primary mood disorder, and not the treatment, the Broaden authors seem to want to have their cake and eat it too. The DBS treatment was designed specifically to alter mood, personality and behavior. When these emotional changes are negative, and DBS recipients report that these serious and very dangerous adverse effects began post-implant, there is reason to think that they are related to the treatment.
The Broaden paper also mentions 27 reported incidents of serious increase in depressive symptoms (among 21 patients, including 13% of patients during the second year). Four people suffered anxiety that rose to a serious level. Once again, the study authors didn’t classify these emotional side effects as adverse reactions to the treatment.
Non-serious adverse events
According to the published report, eight patients experienced an increase in depressive symptoms deemed “non-serious.” Another 15 people had at least three to five periods of “non-serious” anxiety.
Among other “non-serious” adverse events, 10 people had “pulling sensation along extension site.” This is from the wires that tunnel from the chest through the neck to the brain implant, and it’s known as bowstringing. (Both Jim and Steve had this condition and neither of them would deem it non-serious. Even after explant, they have experienced pain related to scar tissue and nerve damage.)
Thirty-two people suffered headaches and 20 people had “post-operative discomfort or pain.” Nineteen had “persistent pain at the implantable pulse generator site or the surgery site or extension” and 11 people had “hearing and visual disturbance.” Five people reported neuralgia. Ten had paraesthesia.
The authors concluded that “No unanticipated device-related adverse events were reported. No episodes of hypomania or mania occurred during the study. There were no adverse neuropsychological effects.”
Once again, the patient accounts by Jim, Steve and Rich provide a stark contrast to the published report. All three had long-term cognitive issues, and I’ve already mentioned Rich’s scary episodes of mania and extreme impulsivity.
Explant data
The Lancet paper mentions that “four of ten early exits chose to have the device fully explanted shortly after study exit.” But the paper fails to report on the total number of trial participants who ultimately had the device explanted, even though, when the study was terminated, the trial participants were given the opportunity to have it explanted for free. How many trial participants chose this option? The published report omits this key data.
The bottom line
St. Jude, the device sponsor, chose to terminate the trial due to low efficacy rates, after their futility analysis revealed a 17% chance of success if the trial continued, and all of the 201 patients approved for enrollment in the trial were implanted. The FDA actually only required a 10% chance of success, a very low bar to clear, for the trial to continue. The Broaden Trial cleared that bar, and despite the trial’s dramatically low efficacy rates, and the many serious adverse effects reported to the trial doctors and to others, including this reporter, the Lancet authors drew this conclusion from their study:
“The negative outcome of this trial should not be interpreted simply as a failure of subcallosal cingulate DBS for treatment-resistant depression. Subsequent studies are merited . . . Importantly, the frequency of long-term response and remission in this and previous studies suggest that this intervention continues to have promise.”
The Ethicists Weigh In
A number of ethicists, and also some doctors that provide DBS, believe that in the world of clinical trial research, this trial of DBS and other such studies of the treatment are among the most problematic. I first pointed out many of the problems in my 2015 MIA article, and since then, many papers have been published highlighting the various problems, including selective reporting of data, investigator bias, experimental flaws, lack of transparency, conflicts of interest and lack of rigorous protections to safeguard patients from harm.
A group at Michigan State University’s Center for Ethics recently published a paper in Science and Engineering Ethics detailing the parallels between current DBS surgeries and historic abuse of psychiatric neurosurgeries (which resulted in regulations in 26 states). They found that many proponents of DBS don’t view it as psychosurgery. The MSU ethicists wrote that “these assumptions are misguided, leave vulnerable patients susceptible to abuse, and should carefully be reexamined.” They note, in their paper, that “efforts to regulate earlier forms of psychosurgery came only after reports of abuse were rampant . . . The new frontiers of psychosurgery are subject to similar abuses . . . Psychosurgery and electrical brain stimulation programs ought to be told as a cautionary tale of procedures that were generally approved of by the medical community, backed by the popular press, and performed by doctors who truly believed they were helping desperate people.”
Their paper cited information from my 2015 MIA article: “As was shown in the BROADEN trial, without full transparency and heightened regulatory oversight, human subjects can be abandoned and abused . . . If even a fraction of what these trial participants claim is accurate, the current regulations and human protection mechanisms have failed them . . . DBS might very well hold tremendous benefit from persons suffering from a range psychiatric disorders, but any advances will be undercut if the current regulatory gaps are not addressed.”
The MSU group made three recommendations: “regulate psychiatric DBS procedures as if they were psychosurgeries under state law; establish a national database for psychiatric DBS trials; and require IRBs to ensure clinical trials provide proper follow-up care by a trained physician before accepting any protocol.”
After the Broaden trial paper was published, I spoke with Laura Cabrera at the MSU Center for Ethics about the published results and their conclusion that the therapy had shown promise. “It truly makes one wonder about scientific objectivity,” she said. “Therapeutic optimism also affects clinicians and researchers, and this is something that in theory the scientific community is supposed to help regulate. Other issues are transparency and conflicts of interest—just look at the disclosure at the end of the paper. It seems premature to do a large, multisite trial with planned enrollment of 201 people randomised at up to 20 sites, before first conducting smaller double blinded sham-controlled trials in one or two sites. The rationale for this large multisite trial is not clear. And there are no clear safeguards regarding patients participating in trials that are discontinued, or issues related to the cost of keeping the implant and related healthcare costs after the trial is completed.”
In response to this criticism, a SJM representative recently told me that “Abbott provides device support for all patients implanted with our system. We work with physicians to understand their patient’s needs and then provide any related continued care and device support.” The representative also said that Abbott is not sponsoring any clinical trials of DBS for psychiatric disorders at this time, and they are focused on “optimizing DBS for movement disorders… and then we will evaluate next steps for additional indications.”
Yet, according to ClinicalTrials.gov, which lists information about ongoing medical studies, St. Jude devices are listed in nine DBS studies, including four in the active or recruiting stage. The site also lists more than 40 DBS-for-depression studies, and 423 other DBS studies for a litany of disorders: for PTSD, bipolar disorder, schizophrenia, OCD, anorexia, obesity, addictions, dementia, Alzheimer’s and numerous neurological conditions.
Postscript: Follow-up with Jim
I received the highly-anticipated Broaden trial paper from Jim. When I first spoke to him, in 2015, he was about to have the device explanted, two years after he received the implant. He wanted it out of his head, and he wanted the trial sponsor to pay for that expensive surgery, which they were offering all trial participants as they geared up to end the trial by year’s end.
Jim and I then spoke the day after he endured a brutal four-and-a-half hour surgery. It was one of the most extraordinary conversations I have ever had. He was so kind and grateful about so many things, including my time and efforts investigating the Broaden trial.
Regarding his participation in the Broaden Trial, he had an astounding capacity for thoughtfulness, empathy and forgiveness, even for the medical providers that featured in the worst two years of his life: his long-term psychiatrist, who told him about the DBS trial, and recommended it, and the UCLA trial doctors charged with his care. Jim’s chief concern was for the fate of trial participants that agree to participate in these risky experimental treatments.
After that, Jim and I kept in touch, sometimes by phone but mostly by email. He often sent interesting medical papers, not just about DBS, but also about new therapies for depression. And we would talk about all kinds of things. Prince. Radiohead. (“My wife really likes the new album, and I think I do too, but music doesn’t reach me these days. I know what doesn’t sound good but I can’t enjoy what should sound good. Oh well.”) Dogs. (In 2016, he had to put his two family dogs down. Later he adopted a dog from a shelter.) And environmental issues. We have also had the occasional group emails with Steve Ogburn, another of the trial participants I had interviewed for the earlier MIA article.
Jim and Steve met online post-DBS, through a private Facebook group for DBS recipients. They became fast friends and allies. In many ways they understood each other better than anyone else. They were among a rare group of perhaps 500 people that have had a DBS implanted for a mental illness. That Facebook group represented an even smaller group of approximately a dozen people that had gone online, searching for other people like themselves.
Jim was always quick to offer moral support and help. When Steve had computer problems, Jim emailed detailed instructions about how to solve it. Since Jim was a lawyer, he also helped Steve cope with the stress of Steve’s legal battle against Stanford, the Broaden trial center where he was given DBS. Steve is still in the midst of a civil suit against the Stanford doctors and the Stanford review board that approved the study. His trial has proceeded slowly, and it’s now in the deposition stage. Jim also helped Steve fill out an FDA Medical Device Report about Steve’s DBS experiences, an onerous task for anyone, especially for two people experiencing cognitive problems, physical pain and the psychic open wound that is severe, chronic depression.
At every time of difficulty, Jim was there to say to Steve and to me: “I’m sorry,” “Hang in there,” or write something funny and upbeat, or, simply, “It’s not brain surgery.” And he was always so thankful for even a short reply to his emails.
About a week after Jim emailed me the Lancet paper, he forwarded me an email he’d written to an Oxford group of researchers called the COMPare team, which investigates misreporting of clinical trials. He expressed gratitude for their hard work and asked them to look into the Broaden trial. His note to me read, “I figure it’s a long shot, but why not try?”
On October 31st, Jim committed suicide. He was 52 years old. His wife Stella called me the following afternoon with the news. I knew that he had created an exit strategy. He’d promised Stella he would never activate the plan when she was away. And in return, Stella, who is a nurse and has seen a lot of suffering and death, promised him that when he was ready, she’d be by his side. It was their own unique, albeit gut-wrenching, coping mechanism for his depression.
Stella met Jim in 1995, in a work situation. “Our eyes met, and I thought, ‘Whoah.’ He was so adorable,” she says. “I could see in his eyes how kind he was. He was such a sweet, generous, wise man.”
In their 22-year relationship, Jim and Stella shared many great times. They also struggled with lows, primarily due to Jim’s depression, which began at college, in the ’80s. Jim was first prescribed a tricyclic antidepressant, which helped for two years. But the depression returned and the next time the tricyclic didn’t help. He started trying other meds, saying, “I went down that typical path.” He was practicing law when the depression became severe. Drugs and behavior therapy couldn’t put a dent in it. In 2000, with Christmas approaching, he made the difficult decision to try ECT.
At Cedars Sinai in LA, he received a typical ECT schedule of 12 ECT doses, three times per week. “He was so confused and had all kinds of cognitive issues. But we were told there would only be temporary amnesia,” Stella says. The standard ECT course didn’t work, so the doctor recommended more ECT. Jim endured 32 ECT treatments that winter. “Once the doctor came out and told me he’d had a prolonged seizure for a few minutes, and they couldn’t stop it. After that, luckily, over time, his cognitive abilities, his ability to work and learn came back. It helps that he was so wise and smart.”
But Jim’s autobiographical memory problems persisted. “He couldn’t remember our wedding. Or his mom’s death, even though he took care of her when she was sick and was at her bedside when she died. He didn’t remember 9-11,” says Stella, which indicates that his difficulties in retaining and remembering new life events persisted long after the ECT treatments. “He wasn’t sure who to trust. He’d ask, ‘How do I know that person? He didn’t even know if he could trust me. The ECT changed his perception of himself. We watched the movie Memento so many times.” Stella says that Jim made attempts to reform the California informed consent process with ECT. “The providers routinely tell lies to patients,” she says. “I think he had PTSD related to it—all the horror of that period.”
Jim and Stella did have some good years post-ECT, but in 2013 Jim’s depression became severe again. “No meds were working, he wasn’t willing to do ECT again. He felt like there weren’t any other options,” Stella says. Jim was going through a medication washout period when his psychiatrist told him about this DBS trial. “The early studies were so promising,” she recalls. “Jim said, ‘I’ll try anything to be better.’ The UCLA psychiatrist was a good man. He told Jim, ‘I’m your psychiatrist now. I’m managing everything.'”
Jim and Stella had no idea that the trial was allowed with an FDA investigational device exemption, and that device companies can bypass many of the typical phase I and phase II steps required for trials of new drugs. They also weren’t aware that Jim was participating in the first double-blind trial of its kind. “Maybe [the device] was at the wrong setting, or maybe it was in the wrong place. But he got so much worse. He had to go on disability. But he had such hope every time he went down to UCLA to [have stimulation settings] adjusted. We read somewhere that it might take a few years. But there were months and months of no communication at all with the trial doctors. The DBS caused so much damage. It was so much worse than the ECT.”
“Then after the first year, the UCLA psychiatrist allowed him to start taking anti-depressants again. He was titrated up very quickly, so he had horrible side effects. By 2017 he was on so many meds, some at twice the max dose. The trial doctors had a moral and professional responsibility to take care of Jim, not just to experiment with him. And UCLA kept billing him for medical care during the trial. They use you as a test subject and then you have to deal with fighting over bills? Look what we gave to you. It’s priceless.
“The truth is the cognitive problems kept getting worse. That was because of the DBS. Every episode of depression kills brain cells. But DBS also impacts so many parts of the brain, not just the ones touched by the [DBS] probes.”
Of all the treatments that failed to curb his depression, and also came with many adverse effects, Stella says, “DBS was the big one . . . He was always talking about DBS. He could spend all day talking about it. It was exhausting. I’d come home from work and say, ‘I can’t keep talking about DBS.'”
With the end of the California summer approaching, the daily grind of depression wore Jim down. But he continued to reach out to help other DBS recipients like Steve, who had suffered adverse effects in the trial. He wrote to clinical trial researchers and to watchdogs, in the hope of protecting future trial participants from harm. And he wrote to me about such concerns, as he did in one email this past fall:
“I am disturbed by the practice and ease with which these clinical trials are conducted. As for the FDA — best look in the pockets of the medical device industry and big pharma. That’s likely where you’ll find them. There is a program being promoted by the Oval Office, called ‘The Right to Try’ which purports to help desperate (vulnerable?) cancer patients get treatment with early experimental research drugs. But the draft legislation also eliminates conflict of interest disclosures and patients’ rights to sue for any injuries associated with treatment. Even for gross recklessness or intentional misrepresentation. But hey, they get the latest and greatest treatments right from the research lab, before it has to go through all the regulations that are supposed to ensure treatment are safe and effective. Don’t get me started. Sadly, I lack the fortitude and energy to be able to ‘pull back the curtain’ on this deep-rooted problem. Such is the intersection of politics and research. Take good care of yourself and those around you.”
His sign-off after sending me and Steve Ogburn the Broaden trial results was trademark: “Hope you’re doing well. I am very grateful to you both for the communication, compassion, support, and understanding you have provided along the way. Thank you. I wish you all the best. Take care.”
At home, the depression storm clouds had blocked out the sun. “He said, ‘I can keep living, but this is not a life,'” Stella says. “He didn’t want to hurt people. His heart was huge even though he was suffering. He took every step to plan a death that would not traumatize people around him. He arranged everything so meticulously. A DNR letter and a note for the police explaining he took a lethal dose of meds of his own free will, that he was donating his brain [to science] and that they only had a matter of hours to get it the mortuary.”
Jim was a remarkable person in so many ways. He created a number of interesting inventions, including one to safeguard people. In order to continue to protect his wish to remain anonymous, I can’t describe these inventions. But his need to remain anonymous highlights the stigma of mental illness, and especially the stigma of choosing extreme, last-resort treatment like DBS.
“Jim’s suicide really hurts,” says Steve Ogburn. “He was my confidante and such a grounding force for me and his death has taken me closer to the edge than ever. He was a very special person and I deeply mourn the loss. I wish I could have been there for him. I truly regret that. He would want his life to have meant something. It did for me. I know how hard the depression had been for him. He was a fighter. He really wanted better oversight to protect people in the future. So they aren’t just lab rats. So they don’t have to through the hell we’ve gone through. He deserved so much more from life.”
“I could have begged him not to do it and he wouldn’t have done it,” says Stella. “I could have delayed it. But I didn’t want to risk him doing it alone. I didn’t want him to die by himself. What if he was scared? Struggled? He had already suffered too much, especially in those last five years. With the Nembutal, it was so peaceful and fast. I just held his hand and his face, so he could see me. Within less than two minutes of taking it, he was gone. It was so peaceful. But I think the world lost its kindest soul.”
* Name has been changed at his request
** Surname not included at his request




Now you know the meaning of corporate health care. The health care actually involves maintaining a healthy corporate balance sheet. Your own health is trivial; perhaps it’s better if the patients die quickly, leaving nobody around to question the value of their treatments.
Report comment
Having seen much of the build-up to the selling of DBS, it’s good to see a little bit of realism thrown into the mix. Here you’ve got another procedure for people who are described in some instances as being treatment (i.e. “medication”) resistant. Electrodes in your brain, and a pace maker in your chest, are not likely to be much of an improvement over a drug, if they are any sort of improvement at all. One should look at the fine print, and carefully explore one’s options. Electrodes in my brain, a pace maker in my chest, an exorbitant tab, and a future of up-dates, and anything but relief. I dunno. Maybe it would be better to try something else. A hobby, for instance.
Report comment
This is stunning, even for me. Corruption plus desperation is a bad, bad combination!
Report comment
Affordable and ethical healthcare? $65,000 to $100,000 for brain and chest surgeries and then $14,000 for new batteries every 5-7 years! I understand that science and medicine need to take some risks, but this sounds to me like a huge waste of time, money and manpower for a poor outcome study. Support groups and grief counseling are what was needed here.
Report comment
So psychiatry killed another kind, decent human being. Decline in mental health almost certainly caused by pails full of psychiatric drugs. Followed up by brain injuries and trauma from ECT. And then more brain injury and trauma caused by DBS.
Why isn’t it evident these people have not one clue what they are doing. They are experimenting on human beings, basically debilitating them and torturing them in the name of so-called science.
Report comment
For someone suffering emotionally, a “placebo” is hope for relief; hope is a powerful force that promotes solutions to real problems with living. For someone suffering emotionally, a “nocebo” is hopelessness for relief; hopelessness is a powerful force that hinders solving real problems in life. Neither drugs nor mechanical implants can solve real problems in life; they hinder solutions and are often powerful nocebos that can promote suicide.
Report comment
Why not give people bits of brightly colored gelatin instead then? Psychiatry is based on a major lie anyhow. Just hand out capsules containing more dried gelatin.
Ta da! The placebo effect. 😀
Report comment
Thank you for bringing this to our, and to the world’s, attention.
We’re all capitalism’s guinea pigs.
Report comment
I find this very imagination of planting electrodes inside a living human brain very offensive. At the same time, I cannot shake off the similarity of the setup between DBS and the Nobel Prize wining research about visual cortex of monkeys by Hubel. I thought Hubel’s method was a high resolution way of understanding the brain functions compared to neurotransmitter POV. Greed was definitely what was driving this kind of medical venture. Maybe some smart people, MD’s and a team of cutting edge neuroscientists were all hopeful to find a breakthrough both in clinical results as well as in higher order neuroscience. How excited they must have been. Psychiatrists and neuroscientist are buddy buddy each other. Another Nobel Prize is just around the corner when the human subjects show positive results.
Except humans are not monkeys, rats, or lobsters.
Report comment
Yes, reminder of the fact the maniac who thought up lobotomies was granted a Nobel…
Report comment
Is it too soon to start discussing what sentences and/or penalties these people should be subject to following their eventual conviction of crimes against humanity?
Report comment
No. It’s not too soon. These people are guilty of murder.
Report comment
Danielle, thank you for this excellent article! There is much to be disturbed about here. I read the study abstract (http://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(17)30371-1/fulltext) and noted the authors concluded “This study confirmed the safety and feasibility of subcallosal cingulate DBS as a treatment for treatment-resistant depression,” in the very sentence after observing “28 patients experienced 40 serious adverse events; eight of these (in seven patients) were deemed to be related to the study device or surgery.” This is troubling given that the abstract is the most read and most influential part of scientific articles.
What I find most troubling is that, as you chronicled, it appears the study investigators have either incompetently measured or fraudulently modified the data regarding adverse effects of DBS. This means the raw data themselves are inaccurate. Therefore, the statistical analyses and conclusions based upon these data are necessarily inaccurate. This includes the analyses and conclusions in this study, and in all subsequent systematic reviews and meta-analyses that include data from this study. It also includes the clinical guidelines that will be drawn up based in part on findings from this study. Inaccurate data such as these have the potential to eventually affect the lives of thousands in society.
We have seen this before in studies of SSRIs in depressed children and it has poisoned the literature and prevented society from accurately understanding the true effects of the drugs. It’s incredible how deep this scientific problem cuts. It’s not just that positive studies are published and negative studies are not (publication bias), or that authors spin their interpretation of the findings to make the results appear more positive, it’s that the very data themselves are fudged. I think it’s time scientific journals seriously consider whether industry-funded trials of medical products are sufficiently trustworthy to merit publication.
I ran a quick news search and found this article on DBS for depression that describes results of the trial: http://nationalpost.com/news/canada/could-an-experimental-brain-surgery-make-you-happier. I’ll end by posting the last three paragraphs, which speak to how these findings have been spun and fit the broader biomedical narrative (FYI, Kathryn is a client who received DBS). In this narrative, a demonstrably ineffective and somewhat harmful surgical treatment for depression is cause for hope, helps destigmatize mental illness, and is part of the march of progress toward revolutionary new medical treatments. Sound familiar?
“For now, DBS remains an experimental surgery. While it is reserved for the most severe cases of depression, Kathryn refers to the power that comes from just knowing that something like DBS is out there, and that other potential new solutions are emerging, such as genetic testing and new medications inspired by the drug ketamine.
DBS, because it’s a surgery, also has the opportunity to further destigmatize mental illness, said Dr. Giacobbe, “by showing people that a physical intervention can help an emotional disorder.”
In the meantime, while we wait, we have hope. And for those struggling, hope remains a most powerful medicine.”
Report comment
Hope is a powerful medicine. But hoping in psychiatry is about as rational as hoping the Easter bunny will save me.
Report comment
Thank you, Danielle, for investigating this, and introducing us to Jim, Steve and Rich. It’s horrific what passes for medical science!
One request, though: Please don’t use the term “committed suicide.” Jim was not a criminal; he committed no crime. Say he ended his life, say he chose to die, say he killed himself, but please don’t use criminal terminology for the act he took. It’s demeaning. Words matter. Thank you.
Report comment
Of course this is just the modern version of lobotomy. So we must fight this and demonstrate proficient use of ANY AND ALL AVAILABLE MEANS! Either we act, or they carve on our brains.
Something I just sent to a friend in email which may be of interest:
I have no objection to disability money being paid out. I think such payouts should be expanded in scope and quantity. We are in the latest stage of industrialization. But our politics is completely driven by scapgoating the poor, plus minorities and immigrants. We are dividing into a two tier society. Psychiatry, Psychotherapy, and the Recovery Movement are simply resurgences of the bogus sciences of Social Darwinism and Eugenics. If we expect our democracy to continue, then we absolutely have to move to Social Democracy.
But I am very critical of disability identities. And generally I feel that access to disability payments is being used to coerce people who are already marginalized into accepting a disability identity. No one should be subjected to such coercion. And in my observation the disability identities are usually flimsy and more the product of abuse, injustice, and social marginalization. Accepting such an identity merely exonerates perpetrators.
It will never change so long as people ask for pity. Psychiatry, Psychotherapy, The Recovery Movement, and Born Again Christianity are all based on pity seeking. Things will only change when people organize and start fighting back.
There is one thing and one thing only which ended slavery in this country, the fact that 180,000 black men refused to be Uncle Tom’s, and instead trained with rifles and bayonets and served in federal uniform. If this had not been so, we would still be practicing slavery today.
Report comment
I skimmed the article, but will try to make time to read the whole thing later. Very disturbing.
One thought I have now is that somebody should report this information to Retraction Watch.
Report comment
I feel DBS is a complicated situation.
My mom has Parkinsons. Shortly before she was diagnosed with Parkinsons, she was sent to p-doc for what my aunts believed was depression. Of course, my mom was prescribed p-meds.
Those p-meds, I believe, induced delusional beliefs which weren’t there before she was on p-meds. P-doc had to “adjust” the p-meds before she “stabilized.”
Ten years later, my mom got DBS. She didn’t behave like the patients described in this article — I do believe that adjustments on p-meds might have played a part in correcting the “chemical imbalance” brought on by DBS–
–but she did become less of a person. Her emotions and personality were blunted. She became childlike and had no capacity to enjoy life or get upset. She’s practically a ghost.
Her neurologist said that DBS saved millions of dollars in healthcare costs.
But my mom is nothing but a shell of a person.
Report comment
A very interesting report. And so sad about Jim. I have only read the abstract to the Lancet article as I don’t have access to the whole article but I am wondering why it took 30 authors to write the article. I know it was multicentre but I don’t think there were any centres in UK were there? yet Keith Matthews from Dundee in Scotland is listed as author.
Report comment
Thanks for all the comments. Bramble, the University of Dundee participated in the study. http://discovery.dundee.ac.uk/portal/en/research/subcallosal-cingulate-deep-brain-stimulation-for-treatmentresistant-depression(bc314c75-a986-4f58-99a6-958938e32b6b).html
And that centre has a long history of use of psychiatric neurosurgeries.
Radiohead, I’m so sorry to hear about your mom. Her post-op side effects sound much like the effects of psychosurgeries, which are still done at many of the same centres that conducted the DBS study: https://danielleegan.files.wordpress.com/2015/09/psychosurgeries_thismag.pdf
Report comment
O brave new world! Can’t psychiatrists get a little more creative? Really. Why not start selling head amputations as a way to relieve symptoms of depression? An effective new treatment! Get rid of that bulbous protrusion on top of the neck that is causing all this trouble! It’s the new wonder cure! The cure for mental illness!
Report comment
“Death Therapy, Bob. It’s a sure cure!” From “What About Bob” – see it!
Report comment
Yes. Thanks for the reminder. I’ve seen it many times. In fact, I was forced to watch it inside a psych ward, which was humiliating beyond description. Death therapy would be really funny if it weren’t also true. The problem is that no psychiatrist will tell you that what they offer is already death therapy.
Report comment
I hadn’t thought of it that way. It’s supposed to be so absurd as not to be close to true. Hard to believe they’d show that movie to involuntary psychiatric inmates!
Report comment
Have you read Peter Breggin’s recent article that psychiatrists see the human brain as a cancer?
Report comment
Excellent journalism, and a heartbreaking story. Much appreciation for keeping on top of this story: it’s amazing to me that if no one looks deeper, these terrible lies and crimes slip by.
Report comment
Steven,
These crimes and lies won’t slip by, Lots of People will Believe them…
Report comment
Remember, the Mental Health System and the Recovery Movement are rooted in the bogus sciences of Social Darwinism and Eugenics. These always work by destroying your honor, nullifying your biography and your personhood. They throw you into an untouchable cast.
The only ways of restoring public honor are by demonstrating the ability to politically organize, and by demonstrating the willingness to take risks in the defense of self and others.
Except for the last year of his life, Martin Luther King Jr. was misunderstanding Gandhi and making an unlimited pledge of non-violence. MLK was an Uncle Tom.
MindFreedom has followed this. We must reject MindFreedom.
Report comment
With the success of deep brain implants for depression they want to try other illnesses.
“From December 2014, seven patients (57 percent women) underwent surgery (in four cases the target was the nucleus accumbens and in three the subgenual area). The most remarkable changes included a progressive improvement in all patients of the social isolation type symptoms and auditory hallucinations. Two patients were dropped off the randomization phase because they presented a noticeable worsening of the symptoms when the generator was switched off. One patient presented complications related to the surgery. The study is currently under analysis and collection of patient outcomes and will be finished in 2017 after one more case is recruited. “
Report comment
A few updates….
I highly recommend the work of author and investigative journalist Jeanne Lenzer. She was recently interviewed about DBS on NPR’s Fresh Air. Transcript here. https://www.npr.org/2018/01/17/578562873/are-implanted-medical-devices-creating-a-danger-within-us
The editor of Neurotech Reports, an industry neurotechnology news and information website, recently wrote an editorial critiquing my MIA articles: http://www.neurotechreports.com/pages/publishersletterJan18.html
He also critiqued my piece, and the work of Jeanne Lenzer, in a recent op-ed on Medium, a publishing blog: https://medium.com/@neurotechwriter/defending-dbs-7ad17113d46c
He made a number of claims and comments about my articles that were inaccurate and misrepresentative, particularly in his MEDIUM piece, including that I provided inaccurate data from the trial results. Hmmm, I thought it was the job of the study authors to provide accurate data and hypotheses? And he provided no data to support these claims. Any thoughts about his editorials?
Jim’s wife sent me his medical records. So far, I haven’t had to the heart to crack open that box.
Report comment
Medical-device-regulators
VNS similar to DBS
“How safe is your medical device? Even regulators don’t really know”
Dennis Fegan had a Vagus Nerve Stimulator (VNS) implanted in 2000.
“…. by 2006, he was repeatedly collapsing and losing consciousness. In July of that year he ended up in an ER after his parents found him passed out in his home.
According to Lenzer, doctors discovered that the VNS wasn’t stopping Fegan’s seizures: it was stopping his heart.
Doctors deactivated his VNS, but Fegan was never able to sue the manufacturer, Cyberonics, because a U.S Supreme Court ruling prevents patients from taking device-makers to court over FDA-approved devices.”
http://www.cbc.ca/radio/whitecoat/how-safe-is-your-medical-device-even-regulators-don-t-really-know-1.4577995
Report comment
Thank you for your very well-written article. This is a very important subject.
Report comment
You really have to look at the last 100 years as the whole picture. They just did mkultra / project star gate on this guy and the other experiment victims knowing this type of implant would not help.
His underlying issue is not depression. Hidden from his diagnosis is his history of brain mutilation with antidepressant medications over 40 different ones. That is probably his main issue. Then he has brain mutilation from ECT. Then he has brain mutilation from mkultra implant. Nothing in his history suggests clear convincing emotional issues like depression. It’s all brain injury. His brain is a rotten mess from prior medical model trickery. His doctor tricked him- selecting him as a patient for brain implant because he knew this guy was a manipulateable dope who kept listening to everything he was told and his brain was sufficiently damaged already to make him a prime mkultra candidate.
They knew the implants wouldn’t be of benefit too- they implanted so many people in MKULTRA and every known implant episode was a failure. There’s never been a success.
Something tells me their experiments had nothing to do with depression that’s the way these things go. What did they really use this guy for a guinea pig for. He thinks all they tested was an implant to treat depression that’s never how they work..
http://www.OregonStateHospital.net
Report comment
This is such a heartbreaking story. Once, upon tiring of the FDA’s lack of real vigilence of BigPharma, I looked into its regulatory role in the medical devices area and found that it was even worse – a wild west! I’ve just recently met up with an old friend whose life has been ruined by toxic metal hip implants – a Johnson and Johnson product now off the market. Though he had them replaced, the heavy metal poisoning has severely compromised his health to the point where he’s not all that sure he wants to live.
If only we were beyond capitalism and into something more humane.
Report comment
Doesn’t seem to be capitalism. Capitalism is a system of exchanging money for service.
These guys want us dead and enslaved. They give us false medicine and mutilate us like clockwork as if there was a eugenics plot to kill everyone but the wealthy elite.
All the doctors and police are employees of the elite and they keep a classified system of power under their belt. This is kept from the public who then misleadingly claim everything being done is to profit rather than kill and control the population.
What if all they actually want to do is make weapons and test them on us under the guise of developing medical care and providing it.
That’s what I am saying: they don’t want to provide medical care they want to use our bodies like we are Guinea pigs and they want to kill 500,000+ a year and damage us in various ways to make us less powerful as a group. Every medical application they test say a hip implant or a brain implant, it’s a failure product but they take the experience to make better weapons on the CIA and DOD side and never provide the technology or experience back to the general public side.
Don’t for a second think the drugs they prescribe to us aren’t failure by design that maim us, reduce life expectancy and kill 500,000+ annually by design. The military doctors who administer this program in every city and state are keeping their identities secret so when we go to the doctor we don’t even know they secretly work for the military and harm us on purpose.
https://www.oregonstatehospital.net/
Report comment
I have no problem with the idea of this, if it works. I have more than once daydreamed about having my prefrontal cortex “fixed”. I grew up reading and watching science fiction. this seems natural to me. (I have simplified my opinions here, for brevity.)
Report comment
Deep Brain stimulation on two anorexia women was in the wrong spot or wrong voltage, or wrong frequency, or wrong method of stimulus ( reactive vs continuous vs when by choice) .https://ca.news.yahoo.com/twin-sisters-debilitating-ocd-dead-apparent-suicide-pact-194855023.html
Report comment
Thanks Markps2. Very disturbing information and there’s so little info about what happened, whether they were under the constant care of a neurologist at the time, whether that neurologist had recently changed their settings, how they got access to a gun, etc, etc.
Even worse, the original 2016 PR story about the surgery they were given in 2015, published in the hospital’s magazine, titled Create: http://cdn2.hubspot.net/hubfs/276703/Denver_DBS_Center_OCD_Article.pdf?t=1460067947382
The PR story makes no mention of where the device was implanted in their brains. And this part: “…Adapting the Mazor Robotics Renaissance Guidance System, a system commonly used for spine surgery, [Dr. David] VanSickle [of Littleton Adventist Hospital] is one of the first surgeouns in the world to perform robot-assisted Asleep DBS surgery, and now performs more of these than any other surgeon in the world. ‘With the robot, we gain higher levels of precision,’ he says. ‘And the more accurately we put hte electrodes in teh first time, the better… outcomes were always good, but the procdure has become more consistent, faster, much less expensive – more mundane really. Yet it’s highly underutilized as a therapy…’
The neurosurgeon’s cavalier comment brings up another important ethical issue: can a robot surgeon be held legally responsible for a botched surgery?
Report comment
So how many public failures do the proponents of DBS have?
Chantal Remeny
DBS operation in 2006 “Ms. Remeny, a 40-year-old physiotherapist from Toronto” https://www.theglobeandmail.com/life/the-on-switch/article18161067/
2009, at the age of 43 , obituary https://www.legacy.com/obituaries/thestar/obituary.aspx?n=chantal-remeny&pid=125450309
Sheila Cook, age 62. Jan 2011
DBS operation (failure) to selective lobotomy https://www.telegraph.co.uk/news/health/news/8279576/British-woman-cured-of-deep-depression-by-pioneering-surgery.html
“It was at this time she was referred by her doctors to the University of Bristol who together with colleagues at Frenchay Hospital, were pioneering a new style of deep brain stimulation.
This worked for a month or two but soon the suicidal thoughts returned. “
Report comment