In a recently published commentary in Psychiatric Times, Ronald Pies and Joseph Pierre renewed their “case for antipsychotics,” citing in particular two “placebo-controlled” studies that they said showed that the drugs improved the quality of life of people diagnosed with schizophrenia. But before Pies and his co-author delved into that literature, they made this assertion: Only clinicians, with an expertise in assessing the research literature, should be weighing in on the topic of the efficacy of psychiatric drugs. They wrote their commentary shortly after I had published on madinamerica “The Case Against Antipsychotics,” and it was clear they had me in their crosshairs.
They wrote:
“We do not believe that armchair analyses of the literature by non-clinicians will answer the risk/benefit question in a humane and judicious manner. On the contrary, we believe that the working with psychotic patients, and appreciating their often profound suffering, is an essential part of the equation. Critics of psychiatry who have never spent time with patients and families coping with the ravages of schizophrenia simply do not grasp the human tragedy of this illness. These critics also miss the deep-seated satisfaction that comes from seeing severely impaired patients achieve remission, and even recovery—in which antipsychotic medication usually plays an important role.
As clinicians with many years of experience in treating patients suffering with schizophrenia, our views on antipsychotic medication are shaped not only by our understanding of the scientific literature, but also by our personal care of many hundreds of patients, over several decades.”
Their message here could perhaps be summarized more succinctly in this way: Whitaker, butt out. Then, in a comment Pies posted beneath his own article, he went a step further in his criticism.
“I am tempted to say that if there were such a thing as ‘journalistic malpractice,’ these critics would be up before the Journalism Board for reprimand.”
Now I, in turn, am tempted to reply to Pies and Pierre that my reporting on this subject already went up before a journalism board for review. In 2010, the Investigative Reporters and Editors Association gave Anatomy of an Epidemic its book award for best investigative journalism of that year. But then I realized that this assertion of theirs needed a more careful response.
They are asserting that review of the “evidence base” for medications should be left up to the clinicians who prescribe the drugs. To a large extent, this is indeed how our society functions. It is the “thought leaders” of a medical specialty who create the narrative of science that governs societal thinking and clinical practices. They are the “experts” who review the medical literature and inform the public what science has revealed about an illness and the merits of drug treatments.
A journalist is expected to report the experts’ conclusions. Indeed, 18 years ago, when I first began writing about psychiatry in any depth, I never imagined that I would write a paper like “The Case Against Antipsychotics.” That does lie outside the usual journalistic task. However, I can easily trace the journalistic path that led me to this end. I would never have started down this road if academic psychiatrists—and the American Psychiatric Association—had fulfilled their public duty to be trustworthy reporters of their own research findings, and exhibited a desire to think critically about their own research. It was precisely because I began reporting about that failure, step by step, that I have ended up trespassing on their turf.
On Becoming a Medical Journalist
The role of a medical journalist can be confusing. I first began writing about medicine in 1989, when I went to work for the Albany Times Union as a science writer, and I immediately had the sense that my job had now changed.
Before that, I had worked as a general reporter for a small newspaper, the Plattsburgh Press Republican. In this position, you cover local politics and business, and you are expected to be skeptical of what you are told. You try to support your reporting with an examination of documents, and while you may rely on interviews to flesh out a story, you are aware that the people you quote have an agenda. They want to present themselves to the public in a favorable light.
But once I became the science writer at the Albany Times Union, I understood that my job was to take complicated science matters and make them both understandable and interesting to a lay public. I was covering the march of science, and the people I interviewed – doctors, physicists, etc. – stood on a societal pedestal. Perhaps they could use a little help in explaining their work to the public, but that was because they were used to thinking and speaking as scientists, which apparently was a rarified language that we mere mortals had difficulty understanding.
My job, it seemed, was to serve as a translator of scientific findings. I could make the difficult science “clear” to the public. I have to confess, I was quite happy to be charged with this task. I have always loved science and the scientific mind, and being a science writer was like being paid to immerse yourself in that world. I thought, this is the best job ever.
At the same time, I hadn’t completely put my journalistic skepticism aside. In the spring of 1991, I wrote a series on laparoscopic surgery, which was being introduced to great fanfare at the time. But rather than write about that advancement, I became interested in reporting on how the introduction of the surgery had been botched, as many surgeons, eager to offer the latest technique, did not get proper training in the new method. This, I documented, had led to a number of patient deaths during routine gall bladder surgeries. That was my baptism into thinking of medicine as both a scientific and a commercial pursuit, with the latter having the potential to corrupt the former.
Over the next seven years, I studied and worked in various non-newspaper environments that, I believe, helped me become more skilled in assessing the merits of a published study, and more aware of the problems with the commercialization of medicine. I spent a year as a Knight Science Journalism Fellow at MIT, and later took a job as Director of Publications at Harvard Medical School.
In that position, I edited a weekly newsletter that reported on research by faculty associated with Harvard Medical School. The newsletter was read by other faculty (and in other halls of science), and thus the stories needed to capture the complexity of their research. Equally important, this was at a time that the idea of “evidence-based” medicine was being introduced. The rationale for this practice was that physicians could be deluded about the merits of their therapies, and thus they needed to have their care guided by science. I took that lesson to heart.
Next, I co-founded a publishing company called Centerwatch that covered the “business” of clinical trials of new drugs. From the outset, CenterWatch was an industry-friendly publication. We wrote about the opportunity for physicians to earn extra income by conducting clinical trials, and we presented clinical trials as an opportunity for patients to gain early access to promising new therapies. Our readers were from pharmaceutical companies, academic medical centers, contract research organizations, and financial institutions that covered the clinical trials industry. In addition to publishing a weekly newsletter and a monthly report, we developed a website that helped pharmaceutical companies find physicians to conduct their clinical trials. Pharmaceutical companies also paid us to list their trials that were recruiting patients.
And then I began writing stories that bit the hand that fed us.
As I learned more about the clinical trials industry, I came to understand that it could best be described as a commercial enterprise, as opposed to a scientific one designed to actually assess the merits of new drugs. Trials were often biased by design; there was selective publication of data that helped promote the drug’s commercial success; and academic “thought leaders” lent their names to this marketing enterprise.
We sold the company in 1998, and that was when I went to the Boston Globe and proposed doing a series on the abuse of psychiatric patients in research settings. Yet, and this is important, at that time I still believed in the larger story of progress that psychiatry had been telling to the public. Researchers, I believed, had discovered that major illnesses like schizophrenia and depression were due to chemical imbalances in the brain, which the medications then put back into balance, like “insulin for diabetes.”
The series reflected both of these perspectives. One part of the series focused on the testing of atypical antipsychotics, and how, among other things, a number of the patients who volunteered in the trials had died, and yet those deaths had not been mentioned in the articles published in medical journals. We wrote about the money being paid to the academic psychiatrists to conduct the trials, and told of specific examples of how that financial influence had led several astray, so much so they ended up either in prison or censured by a medical review board.
Another part focused on studies in which antipsychotics had been abruptly withdrawn from schizophrenia patients, with researchers then tallying up how frequently they relapsed. We said this was unethical, since the drugs were understood to be “like insulin for diabetes.” Who would ever conduct a study that withdrew insulin from a diabetic, and then counted how frequently their symptoms returned?
As I have written before, that would have been the end of my reporting on psychiatry, except for the fact that, just as the series was being published, I came upon two research findings that belied that story of progress. And that made me wonder whether there was a larger story to be told.
On to Mad in America
The research findings were these. First, the World Health Organization had twice found that schizophrenia outcomes were much better in three “developing” countries than in the United States and other developed countries. Second, Harvard Medical School researchers had reported in 1994 that schizophrenia outcomes today were no better than they had been a century earlier. It was then that I asked myself a new question, one that could be said to have arisen from my schooling in “evidence based medicine” while I was director of publications at Harvard Medical School.
Was it possible that psychiatry, as an institution, was deluded about the merits of its therapies? The conventional history of psychiatry tells of how the introduction of Thorazine into asylum medicine kicked off a psychopharmacological revolution, a great leap forward in care. But if one dug into the “evidence,” both historical and scientific, did it support that conclusion?
In Mad in America, I reported on a trail of history and science that contradicted the conventional wisdom. The book might be best described as a counter-narrative. It told of a medical profession that, for various reasons, had become committed in the 1960s to telling a story of how helpful the new antipsychotics were, and how that commitment grew stronger when DSM-III was published in 1980 and psychiatry adopted its “medical model” for diagnosing and treating mental disorders. After that, the American Psychiatric Association’s public pronouncements about the biology of mental disorders and the efficacy of psychiatric drugs turned into a full-bodied PR campaign, and academic psychiatry was—in the language of institutional corruption—”captured” by the pharmaceutical industry. Academic psychiatrists, starting in the 1980s, began working for pharmaceutical companies as speakers, advisors, and consultants, and once this “economy of influence” developed, these “thought leaders” told a story to the public that, again and again, pleased their financial benefactors.
There were many parts to that “counter-narrative,” but here are three such examples.
- I wrote that the simple dopamine hyperactivity theory of schizophrenia really hadn’t panned out, and that in the early 1990s, a leading psychiatrist in the United States had concluded that the hypothesis “was no longer credible.” This was at a time that the American Psychiatric Association was regularly informing the public that “we now know” that major mental illnesses like schizophrenia and depression are caused by chemical imbalances in the brain.
- I wrote that the drug-withdrawal studies cited by psychiatry as proving that antipsychotics provided a long-term benefit were flawed, as they compared drug-maintained patients to drug-withdrawn patients (rather than to a true placebo group), and it was well known that once schizophrenia patients had been on antipsychotics, they were at great risk of relapse if they abruptly stopped taking the medication.
- Based on documents that I obtained through a Freedom of Information request, I wrote that FDA reviewers of the risperidone and olanazapine trials had concluded that they were biased by design against haloperidol, and that the trials did not provide evidence that these new antipsychotics were safer and more effective than the old drugs. This was at a time that the atypicals were being touted by academic psychiatrists, in their pronouncements to the press, as “breakthrough medications.”
Now, in a sense, I was climbing out on a journalistic limb here. The history in Mad in America told of a profession that was deluded about the merits of its own therapies, and had practiced—as the book’s subtitle said—“bad science,” which in turn led to the “enduring mistreatment of the mentally ill.” This did not endear me to a number of people in the psychiatric establishment, but in the years after Mad in America was published, what did we subsequently learn?
- The chemical imbalance theory had in fact failed to pan out by this time. As Pies memorably wrote in a 2011 blog, “the chemical imbalance theory was always a kind of urban legend, never a theory seriously propounded by well-informed psychiatrists.”
- In 2002, shortly after Mad in America was published, psychiatrist Emmanuel Stip wrote that when it came to the question of whether antipsychotics were “effective” over the long-term, there was no “compelling evidence” on the matter. The relapse studies did not provide such evidence.
- In trials conducted by the NIMH and other governmental agencies, the atypical antipsychotics were not found to be superior to the first-generation antipsychotics, which led Lancet to pen this memorable editorial: “How is it that for nearly two decades we have, as some put it, been ‘beguiled’ into thinking they were superior?” Lancet wrote that in 2009, seven years after Mad in America was published.
In short, I had followed a journalistic path—asking questions and searching through documents—to tell a history in Mad in America that countered what psychiatry, as an institution, had been telling the public about chemical imbalances and telling itself about the “evidence base” for its medications. And here’s the point: If academic psychiatrists had been telling the public that the biological causes of major mental disorders remained unknown, and that the relapse studies did not provide good evidence that antipsychotics provide a long-term benefit, and that the atypical trials were biased by design against the old drugs, then I would not have had much of a book to write. It was psychiatry’s own failures, in its review of its science and its communications to the public, that invited a journalist to challenge its “ownership” of this story.
On to Anatomy of an Epidemic
In Mad in America, I had explored the thought that antipsychotics worsened long-term outcomes. This is obviously a question that any medical specialty ought to ask itself—how do its therapies affect patients over longer periods of time—and yet it was not a question that psychiatry, as an institution, had answered. It had relapse studies that it pointed to as evidence that antipsychotics needed to be taken on a continual basis, but I couldn’t find any instance where psychiatry had compiled an “evidence base” that these drugs—or any other class of psychotropics—provided a long-term benefit.
That was the hole in psychiatry’s evidence base that I sought to fill when I wrote Anatomy of an Epidemic. And in this book, I simply sought to put together a narrative of science, from psychiatry’s own research, that could best provide an answer to that question for the major classes of psychiatric medications. I was indeed stepping outside the usual journalist’s role in taking up this task, but I sought to do it only because of psychiatry’s failure to address this issue in any substantive manner.
As I had already learned from writing Mad in America, putting together such a narrative requires that you push past the abstracts and discussion sections in published articles to focus on the data. Anyone who has spent time reading psychiatry’s scientific literature will discover that, if the data is unfavorable to the drug, you often can’t rely on the abstract for an accurate summation of findings, and that the discussions can lead you astray. The abstracts may be spun to downplay the poor results, and the discussions may gloss over the poor results, or seek to explain them away. Often, the data itself—if the results are poor for medicated patients—may be presented in a confusing fashion. You have to learn how to deconstruct the study, based on the data that is presented, to best understand the results.
This type of spinning and obfuscation can be seen in many NIMH-funded stories. For instance, in the STAR*D study, which was touted as the largest antidepressant trial ever conducted, the investigators promoted the notion that two-thirds of the 4041 patients that entered the trial eventually remitted. If a patient’s first antidepressant didn’t work, try another, and eventually an antidepressant will be found that does work. However, nothing like that actually ever happened in the study. Only about 38% of the patients ever remitted (according to the criteria set forth in the protocol), and the graphic that presented the one-year outcomes defied being understood. It took years before an enterprising psychologist, Ed Pigott, figured out that graphic, which told of how only 108 of the 4041 patients had remitted, stayed well, and in the trial to its one-year end. Thus, the documented stay-well rate was 3%, which is a far cry from the 67% remission rate promoted to the public.
Similarly, in the TADS study, the investigators made it appear that just as many suicide attempts had occurred in the non-drug groups as in those exposed to Prozac, which was the conclusion suggested in the abstract. In fact, 17 of 18 of the youth that had attempted suicide had been on the medication. In the MTA study of ADHD treatments, you had to read very closely to see that medication use was a “marker of deterioration” at the end of three years, and that at the end of six years, the medicated children had worse outcomes.
In sum, as I said at the beginning of this post, I agree that writing a paper like the Case Against Antipsychotics is outside the usual domain of a journalist. Pies and Pierre are right about that part. But the only reason I came to write in this way about psychiatry is because psychiatry, as an institution, so obviously failed to fulfill its scientific duty to the public. This institutional failure was an important part of the journalistic story I sought to tell in Mad in America, Anatomy of an Epidemic and most recently in Psychiatry Under the Influence, a book I co-authored with Lisa Cosgrove. It’s not that I set out to trespass on psychiatry’s turf and write reviews of the “evidence base” for its drugs. Indeed, from a journalistic perspective, I am reporting on how the scientific literature contains a story, regarding the long-term efficacy of its drugs, that psychiatry is not willing to tell to the American public (or to itself.)
Deconstructing the Quality of Life Studies
With this context in mind, we can now turn to the claim by Pies and Pierre that there are two well-designed, placebo-controlled trials that showed that antipsychotics improve the quality of life of schizophrenia patients. This claim can provide a test case of what I have been writing about here: Do we see, in their reporting on the study, the critical thinking our society would like to see in those who tell of the “evidence base” for psychiatric drugs? Or do we see yet another example of how leaders in American psychiatry, in their communications to the public and to their peers, draw a conclusion that will support what they want to believe and support their clinical practices, even when such a conclusion is not supported by the data?
In short, we will want to see whether their interpretation of the study reveals a devotion to using clinical research to improve patient care, or using it to boost a belief in their profession.
S. Hamilton, et al. (1998). Olanzapine versus placebo and haloperidol: quality of life and efficacy results of the North American double-blind trial. Neuropsychopharmacology 18: 41-49.
Authors: This is a study that was conducted by Eli Lilly as part of the trials it conducted to get Zyprexa approved by the FDA. The study was authored by employees of Lilly Research Laboratories.
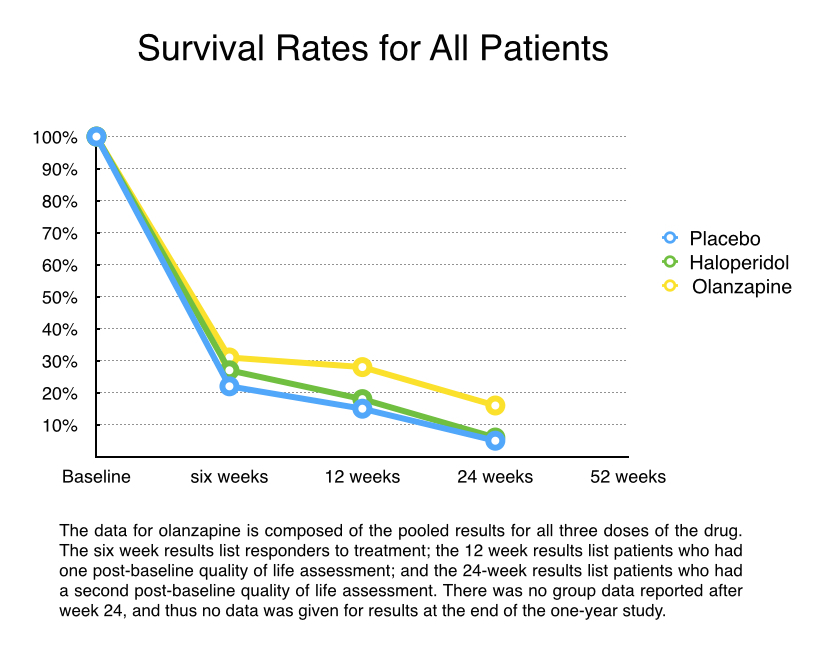
Methods: Investigators at 23 clinical centers recruited patients with a diagnosis of schizophrenia, ages 18 to 65, into the study. The patients were mostly a chronic group, with a mean age of around 36. After being enrolled, they were hospitalized and abruptly discontinued from their antipsychotic medications. They were kept off such medication for four to seven days, and any “placebo responders”—those who got better during this period—were washed out of the trial. Those who were suffering from an “acute exacerbation” of symptoms at the end of that washout phase were randomized into one of five treatment groups: placebo, three olanzapine groups at different dosages, and haloperidol at a daily dose of 15 mg. All patients at randomization were given a Quality of Life Score (QLS), which became their “baseline” measurement for assessing later changes in their quality of life.
All volunteers were hospitalized for the first two weeks, and then were discharged during weeks two to six if they “responded” to treatment (a 40% decrease in psychotic symptoms). At the end of six weeks, “responders” were entered into an extension study designed to last another 46 weeks, with their quality of life assessed at weeks 12, 24, 36, and 52. The researchers hypothesized that those treated with olanzapine would show superior improvement in their QLS scores than the placebo patients, with this benefit persisting throughout the year-long study.
Results: There were 335 volunteers randomized into the five treatment groups (67 each). Only a minority of patients (28%) responded to treatment in the first six weeks and continued into the extension part of the trial. Of the 95 patients who entered the extension trial, only 76 survived another six weeks and thus had one post-baseline QLS store (at week 12 from start of study). There were only three patients in the placebo group, 4 in the haloperidol group, and 33 in the three olanzapine groups who stayed in the trial to week 24 and thus had a second QLS assessment. There were no further detailed reports of QLS scores because so few patients stayed in the trial past the 24-week mark.
To calculate QLS scores, the Eli Lilly investigators used a Last Observation Carried Forward (LOCF) score for those who survived until week 12 but then dropped out before week 24, and then added this LOCF data to the 24-week scores for the 40 patients who stayed in the trial to that point. The Lilly investigators reported these findings:
- The olanzapine patients who responded to the drug during the first six weeks had much better QLS scores at 24 weeks than they did at baseline.
- The olanzapine responders showed significantly more improvement in QLS scores at the end of 24 weeks than the placebo responders.
- There was no significant difference in improvement in QLS scores for the olanzapine and haloperidol responders.
Conclusion in published paper: “Improvement in quality of life was observed in olanzapine-treated responders.”
My interpretation
a) The study is unethical.
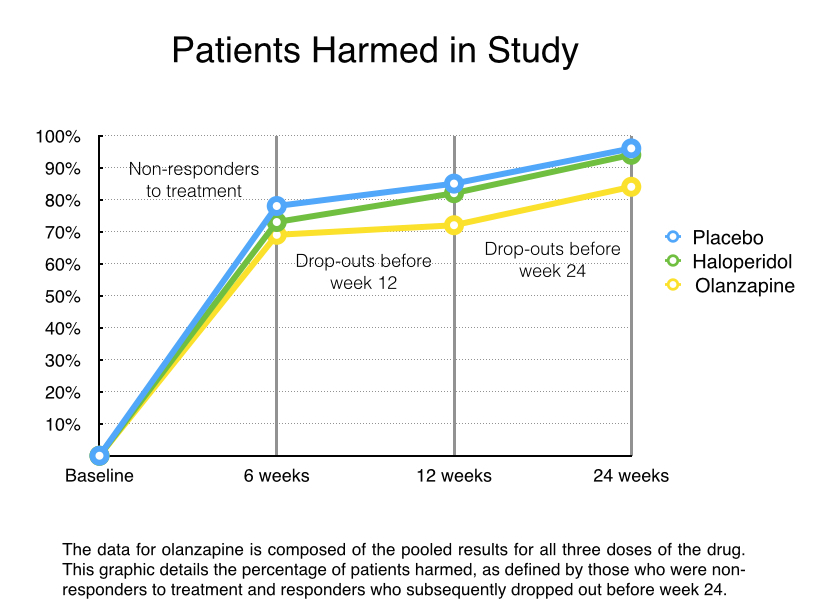
As psychiatry regularly informs the public, schizophrenia patients who abruptly stop taking their medication are at great risk of suffering a severe relapse, which puts the person at high risk of suicide. There is also worry that after such a relapse, a person may not regain the same level of stability following the resumption of medication. In this study, the abrupt discontinuation was expected to lead to an “acute exacerbation of symptoms.” Then one-fifth of those patients would be left untreated and go through weeks of withdrawal symptoms.
Thus, all 335 patients randomized into the study were exposed to harm (abrupt discontinuation of their drugs), and 67—the placebo group—were exposed to extended harm.
All told, 240 of the 335 patients (72%) failed to respond to “treatment,” and thus could be counted as harmed in the study. In addition, 55 of the responders then dropped out before week 24, and given that they were in clinical care at the start of the study, this drop-out result would seemingly tell of harm done. Thus, 295 of the 335 patients who entered the study (88%) either failed to respond to treatment or dropped out of care by week 24.
The investigators did not report on adverse events or suicides, and they provided no information of what happened to non-responders following week six. However, when I reported on the atypical trials for the Boston Globe, I discovered that 12 of the 2500 volunteers in the olanzapine trials had died by suicide. If that rate held true in this study, one or two of the 335 volunteers would have killed themselves, their deaths properly attributed to a study design that put all of the volunteers in harm’s way.
b) The study is biased by company authorship.
This was a study of olanzapine conducted by Eli Lilly: it designed the study, analyzed the results, and reported the results. It was a study designed to produce a marketing blurb, which is that its drug produced a Q of L benefit over haloperidol and placebo.
c) The study is a failed study.
The endpoint for this study was quality of life at the end of 52 weeks. However, there were so few volunteers who made it past week 24 that no results were reported after that date. Thus, the study failed to provide evidence that olanzapine provided a Q of L benefit that persisted, as had been hypothesized.
d) The study is biased by design against “placebo.”
The patients randomized to “placebo” were going through abrupt withdrawal from antipsychotics, and were then left untreated for withdrawal symptoms. Given what is known about the hazards of abrupt withdrawal, this “placebo” group could be expected to fare poorly.
This design lies at the heart of psychiatry’s self-deception, and the gaping hole in its evidence base. Pies and Pierre write in their paper of focusing on “placebo-controlled studies,” as such studies are seen as the gold standard in clinical research. But psychiatry has very few true “placebo-controlled” studies in its research literature. What it has is an abundance of studies where patients abruptly withdrawn from their medications are dubbed a placebo group, which means they masquerade as a placebo group. This is psychiatry’s dirty little secret, and like all dirty little secrets, it is conveniently kept hidden from the public, and as this hiding goes on and on, psychiatry convinces itself it really has “placebo-controlled studies” that it can cite.
e) There is no evidence that olanzapine improved the quality of life for patients diagnosed with schizophrenia.
The baseline QLS score in this study was taken when patients, having been abruptly withdrawn from their medications, were randomized into the treatment groups. Thus, the baseline score told of Quality of Life when the patients were suffering from a withdrawal-induced exacerbation of symptoms. This set an artificially poor baseline score.
Furthermore, to assess whether a drug improves quality of life for a group of patients, it would be necessary to collect QLS scores for all of the patients randomized into the study, and do so at all of the scheduled assessments (weeks 12, 24, 36 and 52). In this study, we know that 69% of the patients treated with olanzapine failed to “respond,” and thus this group could be expected to have a poor Q of L score. Another 15% of the olanzapine patients dropped out before week 24, and thus we might imagine that their quality of life was not so terrific at that point. In order to draw any conclusion about the effect of antipsychotics on quality of life in this study, even in relation to the artificially poor baseline score, you would need to report the scores for all patients.
In short, the results reported in this paper are for a small, select group of good responders to olanzapine (16% of the initial patients), and not for all of the patients treated with olanzapine.
f) The bottom-line results
From a scientific perspective, the design set up this comparison: in patients who had been ill on average about 9 to 10 years, the study compared outcomes for those who were abruptly withdrawn from their medications and then put back on an antipsychotic to those who were withdrawn and left untreated for that withdrawal. The study found that there was a higher response rate for those placed back on olanzapine, and also found that in a select group of olanzapine responders, their Q of L scores had improved notably in comparison to when they were going through abrupt withdrawal. At the same time, the study found that chronic patients withdrawn abruptly from their medications and left untreated fared poorly, as could be expected.
H. Nasrallah, et al. (2004).Health-related quality of life in patients with schizophrenia during treatment with long-acting, injectable risperidone. J Clin Psychiatry 65:531-36.
This study suffers from all of the same defects as the olanzapine study, plus one. The study was funded by Janssen, the manufacturer of risperidone. Most of the authors of the study were Janssen employees, and the lead author was psychiatrist Henry Nasrallal, who disclosed he had financial ties—including serving on speakers’ bureaus—for Janssen and a number of other pharmaceutical companies. A 2014 “Dollars for Docs” report by ProPublica showed that Nasrallah had been paid by 14 pharmaceutical companies for some service or another from August 2013 to December 2014.
The volunteers recruited for the study—a slightly older, more chronic group than the patients in the olanzapine study—were abruptly withdrawn from their medications. It appears that “placebo responders” were then washed out from the study, although this is not clearly stated. The 369 patients were randomized to placebo, or to three groups given different dosages of injectable risperidone (along with an oral dose of risperidone for the first three weeks).
As was the case with the olanzapine study, the placebo group was composed of chronic patients exposed to the hazards of abrupt drug withdrawal, and left untreated for those symptoms.
The study authors do not provide any details about the fate of all 369 patients. There is no information on study dropouts. The authors simply report that at the end of 12 weeks, quality of life had deteriorated in the placebo group, and had improved remarkably in the three risperidone groups. Patients given a 25 mg. dose of injectable risperidone were reported to be enjoying a quality of life at the end of 12 weeks similar to the general U.S. population, with their mental health now just as good as the average Joe’s. The chronic patients, it seemed, had been restored in 12 weeks to near physical and mental “normalcy,” a result so remarkable it reminds one of stories from the Bible.
Pies and Pierre Report the Results
In their article, Pies and Pierre assert that only caring clinicians, such as themselves, are capable of evaluating the evidence base for their patients. Journalists who dare to do so should be seen as guilty of journalistic malpractice, and given that I have now offered my opinion on the merits of these two studies, I imagine they would want me reprimanded anew.
For their part, Pies and Pierre informed readers that in “both studies, patients treated with the antipsychotic showed significantly greater improvement in Q of L than those treated with placebo.” Moreover, they wrote, “Nasrallah et al found that long-acting risperidone (25 mg.) improved Q of L to levels not significantly different from normal.”
And thus the relevant question for society: Does their review of the evidence in this case instill confidence that they can be trusted as the keepers of the evidence base for psychiatry? Or do we see the very type of assessment that made me skeptical about this medical specialty in the first place?















Ah, credentialism…the last refuge of the scoundrel. These psychiatrists are so consumed by their inferiority complex — like Rodney Dangerfield, they get no respect from the other medical specialties — they leap at the chance to lord it over a journalist, even as the evidence against antipsychotics mounts. For all their preening, these guys are industry hacks, and their “scientific” bona fides are on the par with tobacco company science that used to tell us that nicotine was not harmful or addictive. For a a fascinating look at what is possible when psychiatrists stay out, Pies & co. should read Suzanna Cahalan’s Brain on Fire. This young woman made a complete recovery from madness because, for once, psychiatrists of all stripes (those representing the drugging establishment as well as the Freudian dogmatists) knew their place, did not muscle their way in (unlike in the Justina Pelletier case), and let the real doctors and real science do their work.
Report comment
GetItRight,
There’s a vested interest among all doctors to diagnose brain illness rather than a psychological situation – because this allows them to earn money as prescribers gives them control.
At the start in 1980, I thought Psychiatrists only talked to people and I asked for talking treatments. After a number of years of unsuccessful chemical treatment I was diagnosed very badly. But I then cut the drugs moved to psychology and recovered. So I was right to begin with.
I have thirty years of genuine recovery now.
Report comment
Fiachra, I love your story and wish it were everyone’s story. You must have had an uncommonly, extraordinarily capable and effective therapist. Lucky you. I myself believe that “mental illness” or whatever passes for mental illness — psychiatric symptoms is a better phrase — can have psychological as well as biological etiology. I just abhor psychiatric drugs and would look for natural healing therapies in addition to psycho-social support.
Report comment
Thanks GetItRight,
I did have a lot of very good social and thereuptic support.
Report comment
We see the very type of assessment that made you skeptical about this medical specialty in the first place.
I’d also love to know why Pies and Pierre think it’s appropriate for the current ‘bipolar’ treatment recommendations to recommend combining the antidepressants and / or antipsychotics. When all doctors are taught in medical school that combining these drugs classes can actually create symptoms which appear to the psychiatrists as the positive symptoms of ‘schizophrenia,’ via anticholinergic intoxication syndrome / anticholinergic toxidrome.
https://en.wikipedia.org/wiki/Toxidrome
And the neuroleptics can also create symptoms that appear to the psychiatrists to be the negative symptoms of ‘schizophrenia,’ via what’s medically known as NIDS.
https://en.wikipedia.org/wiki/Neuroleptic-Induced_Deficit_Syndrome
And since neither of these iatrogenic, neuroleptic induced syndromes, which mirror the positive and negative symptoms of ‘schizophrenia,’ are billable DSM disorders, out of sight out of mind, they are no doubt almost always misdiagnosed.
How do Pies and Pierre distinguish between these neuroleptic induced syndromes and the so called ‘real schizophrenia’? Because I know none of my psychiatrists were able to do this. Thankfully, after researching medicine for 10 years and pointing out to a doctor with a brain, that psychiatric drug induced anticholinergic toxidrome poisoning was neither ‘bipolar,’ nor ‘schizophrenia.’ I was finally able to get those misdiagnoses / defamation of my character off my medical records.
And I will mention that another historic ‘dirty little secret’ of the psychiatric / psychological industries is that those industries have been profiteering off of covering up child abuse and easily recognized iatrogenesis for ‘the two original educated professions’ for decades, according to an ethical pastor of mine. And this would, of course, give insight into why ‘the prevalence of childhood trauma exposure within borderline personality disorder patients has been evidenced to be as high as 92% (Yen et al., 2002). Within individuals diagnosed with psychotic or affective disorders, it reaches 82% (Larsson et al., 2012).’
Report comment
I made this point below. We cannot even trust that these studies are comparing comparable groups, the reason being that schizophrenia as a diagnosis lacks validity.
Report comment
Ditto.
Report comment
I agree, BPDT, as does Dr. Thomas Insel, the former head of the NIMH. So why are we, as a society, still utilizing the DSM for “mental health” “diagnosing” and billing? The sad truth is, the DSM is a classification system of the iatrogenic illnesses created with the psychiatric drugs, and of course the medicalization of the human condition, so people can be railroaded into the psychiatric system.
Report comment
A professional who commented on Dr. Pies’ article stated that he could attest to the fact that quality of life declines precipitously during the time patients come off their medications. He contended that if those in a placebo group are suffering from withdrawal during the course of a study, that could skew results considerably and asked if any studies compared outcomes with medication naive groups or subgroups.
Dr. Pies answered that there are no such QOL studies that he is aware of. He said such issues could be dealt with by very slow tapering (usually 3-6 months). But he also claimed that “supersensitivity psychosis” because of withdrawal is speculative, and he dismissed the commentator’s observations by saying the concept of a withdrawal effect is only theoretical. Of course, in his article he claimed a superior ability to judge the effects of psychiatric medication because of his work with patients. So apparently anecdotal evidence is important, but only if it doesn’t contradict Dr. Pies.
Report comment
Marie,
It’s the story of the continuing mistreatment of the “mentally ill”.
In my opinion there’s nothing speculative about ‘supersensitivity syndrome’ – if I hadn’t learned how to carefully withdraw from drugs 30 years ago, I’d still be ‘Care in the Community’ in Ireland Today.
Report comment
“In my opinion there’s nothing speculative about ‘supersensitivity.”syndrome.”
Interestingly, mainstream psychiatry recognizes other withdrawal effects; for example, delirium due to alcohol withdrawal. They refuse to acknowledge the effect of withdrawal from psychiatric drugs because they peddle them.
Report comment
Hi GetItRight,
I think its well known that withdrawing anything that relieves “anxiety” will result in worse “anxiety” than was there to begin with.
Report comment
I’m ‘antidotal’ evidence people suffer from ‘drug withdrawal induced super sensitivity manic psychosis,’ even after, according to my medical records, being ‘properly’ weaned from the drugs for years, not months. So, I’m quite certain, Pies is wrong.
The reality is the psychiatric industry generally does not want to wean people off the drugs, and so they do not currently know how to do so properly, period.
And like Whitaker points out, one’s brain acquires too many dopamine receptors, to compensate for a dopamine antagonist / neuroleptic / antipsychotic, because brains are neuroplastic, and do try to compensate for the harm being done to them by the drugs.
But this compensation by the brain being attacked by neuroleptics, can result in a drug withdrawal induced manic psychosis, when one is weaned off the drugs, even over several years. And this withdrawal induced side effect can occur at a much later period of time than today’s psychiatric industry, which does not have any financial motives to study how to wean people off of drugs at all, and has no financial desire to research such, believes.
The reality is today’s psychiatric industry is only interested in research that proves their drugs are ‘wonder drugs.’ And has done next to no research into how to wean people from their drugs, so has next to no expertise into how to heal people from the harms of the psychiatric drugs.
I absolutely agree that when a medical specialty becomes so self-intersted and profit only motivated, it is the journalists’ duty to investigate such a corrupt industry. Thank you from the bottom of my heart for the work you’re doing, Robert Whitaker.
Report comment
If you’re pushing dope, it’s better if your stuff is addictive. That way the customer always comes back for more.
Report comment
.`..he claimed a superior ability to judge the effects of psychiatric medication because of his work with patients. So apparently anecdotal evidence is important, but only if it doesn’t contradict Dr. Pies. SPOT ON!
Another problem is that psychiatrists will DIAGNOSE the `schizophrenia’ etc but since they spend no more 10 minutes at a time with most of their patients, it could be said that that SEE them but they don’t actually KNOW them at all. I wonder how many times Dr Pies has sat with a patient over a period of hours per day for several weeks? I have, and they were some of the most wonderful, insightful and courageous, `severely impaired,’ `profound[ly] suffering’, `the ravages of schizophrenia’, people I have ever known. `Critics of psychiatry who have never spent time with patients and families coping with the simply do not grasp the human tragedy of this illness.—in which antipsychotic medication usually plays an important role’ in maintaining a dreadful QOL. I suggest most psychiatrists belong more to those who never spent time with these suffering people partly because they write them of as incurable, chronic uninteresting detritus to be drugged to a standstill and not bother anyone, particuar
ly them.
Report comment
Yes. Plus if they spent too much time with us they might realize that we are human beings. That would make it more difficult for them to torture and defame us.
Report comment
One thing about these two psychiatrists that’s certain – they have not honesty, nor humility, nor shame.
Thank you, Robert, for your dedication to the truth.
Report comment
Psychiatrists are biased. They don’t want to believe their beloved treatments do any harm. How about talking to those of us whose lives have been ruined by anti-psychotic drugs? We know firsthand the harm they cause.
Report comment
Spot on Robert! As a PhD student in nursing, it is common knowledge that all research studies are not without bias. These two psychiatrists only offer the research findings to reinforce their argument. That’s the game, isn’t it? It’s the same for evidence-based medicine or evidence-based practice: if the evidence is poor, then the outcomes will be poor!
Report comment
“But psychiatry has very few true “placebo-controlled” studies in its research literature. What it has is an abundance of studies where patients abruptly withdrawn from their medications are dubbed a placebo group, which means they masquerade as a placebo group.”
Wow! Wow.
Liz Sydney
Report comment
“Journalistic malpractice”, if there were such a thing, as far as I’m concerned, would be a matter of a journalist trying to pass fiction off as a news story, that is, blatantly lying. The half-truths and innuendos of people who are supposed to be medical professionals is another thing altogether. Typically, if the shots were intended for you, they miss entirely as these studies they come up with are practically irrelevant to the case you are making. What have we got here? Two basically short term drug company funded studies of chronic mental patients. This research has to have very little to do with how well people fare long term off or on psychiatric drugs, as it was only looking at the short term, and to the neglect of any patient never exposed to such drugs.
Canadian psychiatrist, and historian of psychiatry, Edward Shorter does something similar with other critics of conventional psychiatry. He dismisses some studies of a psycho-sociological nature as examples of 1970s agenda. He claims that some studies, and histories, too, are based on a lack of understanding of medicine. He would have historians trained in medicine. Not so much of a problem, except in so far as it is probably absolutely unnecessary when it comes to developing such an understanding, and also, as in his case, it could mean a conflict of interest. “Conflict of interest?,” you say. Yes, the lauded author of a history of psychiatry is also a professor of psychiatry. I don’t see how that couldn’t, in some sense, have a negative effect of his sense of objectivity.
http://dhayton.haverford.edu/blog/2013/07/18/a-conversation-with-edward-shorter/
Report comment
You should see his treatise on ECT. He and St David Healy join forces for one of the worst, most biased, pieces of pseudoscience you will ever see.
Report comment
Great work, as always, Bob. Being sneered at by these guys is the ultimate compliment.
I’d love it if you could comment further on the “Placebo washout” approach described so clearly in the first study. It appears that they systematically removed anyone who got better when coming off antipsychotics. This always seems like a pretty sleazy maneuver to me. If you have people whose quality of life IMPROVES when they STOP the “treatment,” wouldn’t that be very important data? I’d love to compare the “washout” folks at 52 weeks to the Zyprexa users at 52 weeks.
All that being said, the most important point is that the study is meaningless, as they couldn’t even record their primary measure due to an incredibly high dropout rate. Not to mention that they only compared the QOL of the RESPONDERS! Isn’t that like saying that “people who experience pain relief when taking aspirin have less pain than those who didn’t experience pain relief?”
And of course, the other huge deal is that they selected out only these two studies as their best evidence to support their point, rather than looking at the whole of the literature. Such “cherry picking” is systematically criticized (and rightly so) when committed by those who disagree with them. Why are they allowed to get away with it?
The real message here is: “Trust us and don’t ask too many questions like that guy over there.” Or “Don’t confuse yourself with facts.”
Thanks for another excellent blog.
—- Steve
Report comment
Steve said: “I’d love it if you could comment further on the “Placebo washout” approach described so clearly in the first study. It appears that they systematically removed anyone who got better when coming off antipsychotics. This always seems like a pretty sleazy maneuver to me. If you have people whose quality of life IMPROVES when they STOP the “treatment,” wouldn’t that be very important data? I’d love to compare the “washout” folks at 52 weeks to the Zyprexa users at 52 weeks. ”
Yes! This was my thought exactly.
Their management of and failure to report on the placebo washout group is nothing short of academic malpractice…and there seem to have been so many of them. Throughout the study, they have conveniently excluded anyone who didn’t respond in a way that could be used to promote their drugs, and no real follow-up or explanation has been given. If a study starts with, say, 400 people, all 400 should in some way be accounted for at the end…including deaths, refusals, etc.
A study should NOT begin after you have excluded a whole bunch of people you recruited and then decided you didn’t like, because their reactions didn’t fit your preferences. It is academic dishonesty.
…and then for Pies et al to revert to anecdotal “evidence” from their years of “caring” for their patients as being superior to any data from any study…well….
Another excellent piece. Thank you Robert
Report comment
Sure. The Zyprexa folks are going to be a lot heavier than the control group.
Report comment
Ron Pies doesn’t indulge in clear thinking. Have a look at some of his attacks on Phil Hickey as well, when Phil took him apart for his famous 2012 claim that `the chemical imbalance’ was an `urban myth’ that nobody important had ever really supported. Of course Phil and many others demolished that, but the most telling thing was that he set himself up AGAIN and was demolished again. This is a thought leader of psychiatry?
Report comment
I am only one guy. But my experience on antipsychotics has been the stuff of nightmares. Rebound psychosis from going off seroquel? Check. Suicidal ideation on zyprexa? Check. Apathy on haldol? Check.
This is infuriating.
Great work Mr Whitaker
Report comment
Oh ya I forgot. I became psychotic because my doc put me on Wellbutrin which induced sixty days of insomnia.
Report comment
I’m surprised you were not offered a benzodiazepine, to which you would now be hopelessly addicted, given that the withdrawal symptoms are worse than those for heroin, are persistent, and can’t necessarily be treated by resumption of the drugs.
Report comment
I was put on Wellbutrin, too, for smoking cessation, not depression. It didn’t work, so I was abruptly taken off of it by my PCP. Then doctors and psychiatrists misdiagnosed the common symptoms of antidepressant discontinuation syndrome, as “bipolar,” a blatant misdiagnosis, according to the DSM-IV-TR of the time.
It seems some of the dumbest and/or least ethical people within society are now working within the medical community. Oh, that reminds me of something that crossed my mind while I was suffering through my drug withdrawal induced manic psychosis. I remember thinking that all today’s doctors were ax murderers in a previous life. And they supposedly were given positions of importance in society by God today, to test them to see if they could be good people when given a blest life. Medical mistakes are the third leading cause of death, guess many are still no better than ax murderers. Now I understand why Jesus supposedly said, all the doctors are going to hell. And I still hope that’s untrue, I’ve found ethical doctors with brains, but there likely will be lots of today’s doctors landing in hell, if there is such a thing.
Report comment
You remain standing. Their artillery tables were incorrect. God alone knows where the barrage impacted.
Report comment
Congratulations; psychiatrists are exposing the bankruptcy of their position when they attack critics rather than respond to criticism! The APA did the same thing with criticism of the DSM by defining problems as “clinically significant.” Your analysis of the data is correct and their criticism is weak- an affirmation.
Best wishes, Steve
Report comment
Only clinicians, with an expertise in assessing the research literature, should be weighing in on the topic of the efficacy of psychiatric drugs.
I did the ultimate research on the topic of the efficacy of psychiatric drugs, I took the stuff and as Anatomy of an Epidemic points out they keep you sick. Zyprexa almost killed me.
Since my recovery I have been working in the addiction field “dual diagnosis” and the stories are almost always the same, when I was a child they said I had ADHD started those drugs that made me feel like crap zoned out speedy then when I got worse they piled on more pills and called me bipolar. Must have heard that from 300 people since I started taking the time to ask.
This new client I met is in for methamphetamine, he seemed like he was doing ok to me upbeat and all and he comes back from the doctor and tells me the doctor said he was bipolar and started him on Cymbalta and Gabapentin.
I gave him the story on psychiatry but like a typical addict he took the crap anyway but WTF how the hell is Cymbalta and Gabapentin going to help someone who enjoys flying around on meth ?? Gee thanks Doc , I have that sick tired drugged feeling all the time so now I really don’t want to do any meth .
Then when he goes home and doesn’t follow through on aftercare he gets to have all the withdrawal reactions no one even bothered to warn him about.
Cymbalta and Gabapentin will make that guy “better” give me a effing break.
Psychiatry more harm then good , I see it like every day.
Report comment
This is another good point: the foremost “experts” on antipsychotic drugs are those who actually have taken them, like myself and The Cat. Why are we not hearing from these people?
Report comment
…and me. Olanzapine (Zyprexa) is EVIL. It creates a living hell and is a nightmare to come off. Since coming off it 6 years ago I haven’t even been close to being psychotic.
Report comment
“..Only clinicians, with an expertise in assessing the research literature, should be weighing in on the topic of the efficacy of psychiatric drugs…”
NO:- Because they can’t be trusted to tell the truth. (At the time) The President of the British Association of PsychoPharmacologists came from the University that managed my Psychiatric Unit + My Former Psychiatrist was on the examining board of the Royal British College of Psychiatrists AND my Records were still Doctored at Source:- Requested Adverse Drug Reaction Warning was intentionally omitted + Extra pyramidal disability was intentionally omitted; + Longterm Recovery as a result of stopping strong medication and moving to The Talking Treatments was intentionally omitted.
Report comment
Olanzapine (Zyprexa) is EVIL. It creates a living hell and is a nightmare
Evil, hell and nightmare are the three most common words used online to describe that poison. That crap made me so sick, when on it it robs the ability to feel pleasure from anything and then coming off it the withdrawal induces all the symptoms of the severe mental illnesses. It was given to me for the minor problems of anxiety and insomnia then withdrawal left me with what they labeled schizoaffective disorder during the blame the victim not the drug part when the victims try and get off it and get sick.
The most insidious diabolical part of Zyprexa / Olanzapine is that outside observers will say the person on it looks “better”. THAT in my opinion is what really makes that drug truly evil. Emotions down to zombie levels, motivation erased without total sedation “better”.
I wish I could do a better job of describing the effects of that wicked drug, I call it a robbery, the official term is anhedonia but words just don’t adequately describe.
Zyprexa is evil on the level of Thalidomide. It should be banned.
Report comment
It gets a bit long in the tooth. What happens to you or me or Captain Spock on drugs is what happens to you or me or Captain Spock on drugs. We don’t become instant drug-induced experts on the drug. And we don’t become sudden overnight insight-sensations about what the drugs do to other people. Almost all subjective drug experiences are best not mentioned in polite conversation unless — UNLESS! — you have a very rare gift for drug discourse. Which most don’t.
So stick to the science in which your self-proclaimed “expertise” disappears, as rightly it should.
Otherwise I have some sympathy for the sentiment.
Report comment
I agree, BPDT, we’re dealing with a debate between “experts” by experience vs. “experts” educated by pharmaceutical industry biased mis-information, whose livelihood depends on pushing these drugs. And keep in mind, “It is difficult to get a man to understand something, when his salary depends on his not understanding it.”
The neuroleptic / antipsychotic drugs are “torture” drugs, just like the UN came out saying in 2013, in the opinion of this person who was forced to take almost every single one of the ‘atypicals,’ prior to finally being withdrawn from them, which was a staggeringly bizarre experience in itself.
Report comment
the cat:
yes, the ADHD gateway to bipolar is common in my experience too; and also these young people start doing opioids (and eventually heroin too) after all the stimulant drugs
Report comment
I also see this frequently with foster youth. I never cease to be amazed that the obvious seems so obscure to those prescribing the drugs.
Report comment
“It is difficult to get a man to understand something, when his salary depends on his not understanding it.”
Report comment
So in other words, the meth user was doing far better on meth than he or she is doing on psychiatric treatment? The only problem there is that buying meth from a meth dealer is not legal. Given that meth can be prescribed as Desoxyn, he/she could get a prescription and live a crime-free life, sustained by the best medicine for his “condition.” It’s worth considering, if Cymbalta and Gababentin have left any part of the individual’s mind functioning and havene’t destroyed the will to live. Those two drugs are the stuff of living nightmares. I’d sooner inject Desoxyn than allow one tablet of either to enter my body orally. Anyone who doubts the wisdom of this should visit surviving-antidepressants.com and read the histories of people whose enjoyment of life, whose ability to remain neutral as opposed to suffering and suffering deeply, has been destroyed by those two pills. The emotional pain is not depression and it’s not psychological angst. It’s a torment much deeper than thoughts and beliefs can access, and is relentless, it seems.
Report comment
Robert: Perhaps you should write a short commentary (reply) to that journal. It might save time however, if you write to the editor first and ask if he/she would consider a reply commentary. Also, it might be worthwhile to get a clinician to be a co-author.
By the way, the following two research studies that have shown the same changes in brain scans in patients who respond to placebos and patients who take an actual drug might also come handy if you do decide to write a commentary (to mention that drugs can work sometimes for individuals because of their placebo effects, etc.).
http://archpsyc.jamanetwork.com/article.aspx?articleid=2443355 http://archpsyc.jamanetwork.com/article.aspx?articleid=2443354
Report comment
I believe its now accepted that the “Atypicals” were not superior to the original drugs. They caused more health problems, shortened life expectancy and were a lot more expensive:-
In 2005 in the UK the “typical” neuroleptic Mellaril was taken off the market “on account” of its heart rythm effects.
Mellaril was replaced with “atypicals” like Seroquel. But the difference between the two drugs was that Seroquel was 50 times more expensive and a lot more dangerous in terms of heart rythm effects.
(Seroquel carried a black box warning in America and was banned in the American military because of its lethal effects)
Report comment
I think the “Atypical” promotion was a type of Professional and Official fraud that siphoned off a lot of money and killed a lot of vulnerable people.
Report comment
I agree, Fiachra.
Marcia Angel’s book:
https://www.amazon.com/Truth-About-Drug-Companies-Deceive/dp/0375760946
Is a great book pointing out the fraud of me-too drugs, including all the so-called “atypical antipsychotics.”
Report comment
Thanks Someone Else,
It’s a big Back Scratching/Money Laundering arrangement:-
“….Angell says, most of the R & D work is done by colleges and universities funded by the government. …”
Report comment
Wow Pies and Pierre just got owned. That is truly embarrassing to have the two studies they cited exposed like this, as industry-funded, dropout-full, withdrawal-biased, scams…
They just keep make it worse for themselves by opening their mouths / uncapping their pens.
Robert, well done with this careful analysis. It’s much easier for you because your professional status and your income don’t depend on the maintenance of myths favorable to corporations which funds the APA, psychiatrists training programs, universities, etc.
I still think you should not kowtow to Pies/Pierre’s use of “schizophrenia” as if there were such a singular disease entity, something Pies even admitted there probably is not in a footnote to his latest essay. The uncertainty of whether there is a valid coherent entity called schizophrenia adds another wrinkle into how to evaluate these studies. How can we even trust that comparable groups are being compared in these studies given that the diagnoses are invalid?
And lastly, let’s get one thing straight: these are tranquilizers, not medications treating a disease. Seroquel and Risperdal are tranquilizers not medications, simple as that. The ord medications only serves the myth that psychiatry wants to sell, i.e. that severe problems in functioning/living are brain diseases. I hope you will start to separate yourself from repeating that euphemism.
Report comment
“They just keep make it worse for themselves by opening their mouths / uncapping their pens.”
Not at all, because their article is being read by mostly psychiatrists and some other mental health professionals, who take everything they say on face value, a tiny percent of which would ever actually come here and read Whitaker’s.
Report comment
Robert, what would an ethical study on antipsychotics look like?
Report comment
After all Mr. Whitaker who are you to challenge Dr. Mengele?
Report comment
Was it Dr. Mengele who blinded Jewish children in Nazi concentration camps?
At any rate there was an MD (certified with a doctorate in medicine) and legally certified nurses who held down screaming children with brown eyes and put blue dye in. Hey, they were “helping” to normalize the children with nice, blue Aryan eyes! The fact that this only blinded them was an unfortunate side effect of the “medication.”
Being a doctor doesn’t absolve one of being a pathological liar and sociopath!
Report comment
Absolutely!
Report comment
Very readable and I appreciate the autobiographical details from local journalism to MIA.
Hope this doesn’t seem like an annoying diversion but
that commitment grew stronger when DSM-III was published in 1980 and psychiatry adopted its “medical model” for diagnosing and treating mental disorders.
Don’t you really mean the “biological” or “bio-medical” model? We were constantly talking about the medical model in the mid-70’s; it had, since Szasz started writing, traditionally referred to any belief in “mental illness” as an actual disease.
The other thing is: Can you provide a working definition of “evidence-based medicine”? It seems like you have two terms: “Evidence-based” (an adjective) and “evidence base” (noun). Is this a pun or play on words, or are we really talking about the same thing with both terms? I.e. “evidence-based” seems to mean “based on evidence.” “Evidence base” seems to refer to the amount and quality of evidence on hand. So you could have something “based on evidence” (depending on how one defines “evidence”) that’s not based on solidevidence, or consistent with other accumulated evidence. (Or maybe I’m just tired.)
Report comment
“Don’t you really mean the “biological” or “bio-medical” model? We were constantly talking about the medical model in the mid-70’s; it had, since Szasz started writing, traditionally referred to any belief in “mental illness” as an actual disease.”
I think you’ve got a really good point here, OldHead, and I don’t think it was adequately addressed by R. Whitaker’s response.
“I think what APA adopted in 1980 when it published DSM III was a “disease model” rather than a medical model, but I guess I was using the APA’s terminology in quotes.”
And what “model”, pray tell, would one oppose to a “disease model”? A “trauma model”?
I’m just wondering, where is the “oppression model”? There used to be a lot of talk about psychiatric oppression. Correct me if I’m wrong, but psychiatry hasn’t stopped being oppressive, has it?
Report comment
Disease model and medical model seem to be the same to me.
Another thing: “Model” of WHAT? When they say “model” it’s often followed by “of mental illness.” So we should consider that as well. ANY “model” of “mental illness” assumes the reality of “mental illness.”
Report comment
In other words, referring to the “medical model of mental illness” actually connotes acceptance of and employs the medical model by presupposing the existence of “mental illness.”
Report comment
I like both oppression model and trauma model, except (I know I’m starting to belabor this) what are they models “of”? What I’m getting at again is challenging the assumption that there is a “thing” that is now called “mental illness” but should really be called something else. This presumes a category or “thing” which has never actually been shown to exist in the first place.
Report comment
As a chemist by training, I have given this a lot of thought historically. By my understanding, a scientific model is an attempt to provide an explanatory mechanism for a phenomenon that is observed. Any good scientist knows that a model is only as good as its ability to predict the phenomenon in question and/or intervene to alter the outcome in predictable ways. Clearly, the “medical” or “disease” model has proven a total and abject failure in both ways.
The “oppression model” would be a means of explaining why people experience intense anxiety or depression or extreme states from the point of view that such experiences are likely a result of experiencing abuse or traumatizing experiences at the hands of another. We would also hope it might suggest ways of intervening to either prevent the oppressive conditions or to assist in analyzing how a person experiencing such conditions might act or be helped to act in order to alter the predicted outcome.
It doesn’t necessarily imply that the phenomenon in question is an aberration or in fact pass any judgement whatsoever about the phenomenon. It’s more like “when people are traumatized, they’re more likely to hear voices in their heads” or “people who hear voices in their heads are likely to have had a history of individual or social oppression.” The model certainly doesn’t require an acceptance of “mental illness” as a concept.
That’s my take on it, anyway. Good discussion!
—- Steve
Report comment
In re-reading this, I’m inclined to think that the Western approach to viewing “mental illness” or anything else having to do with human beings from a detached, analytical perspective is what creates a lot of the problem. People aren’t “problems” or “hypotheses.” Maybe being “scientific” takes the humanity out of the equation.
Report comment
There is no “reality” to “mental illness”. I’m not that fond of trauma theory, of course. I just see professionals using “trauma” to explain why some folks don’t “recover”, or aren’t “resilient”, and other folks, they would claim are more “normal”, are. I believe “trauma theory” is pretty much that explanation. This is amusing, somewhat, as the predominant “treatment” given for “mental illness” labels is “trauma”, and so many people aren’t quick to abandon their trauma providers. Actually, “trauma” is a more of a psychodynamic (talk therapy) explanation of sorts because so much of this “trauma” is psychological “trauma”, and psychological “trauma” is literally not “trauma”. Sure, perhaps “injury” makes more sense than “disease”, however ‘healing psychological trauma’ is an illusionists trick, and must be right up the shaman’s alley. What am I saying? Bluntly, injury is physiological, or it isn’t injury.
Report comment
psychological “trauma” is literally not “trauma”.
Ugh.
Report comment
Wow, this is *literally* an entirely different reality than mine. Not that it’s only mine, I know others share in the knowledge of the power of psychological abuse, injury, and wounding–as well as the healing of it. And I can accept and respect that this is not your belief or position, Frank, from what you state above. I see that this is your truth.
But I am so struck at the diversity of beliefs and realities that exist on the planet, even just on this one website. Whether people agree or not on basic items such as this, can they/we still co-exist peacefully? That would be my question at this time. Can we respect diverse realities, rather than to challenge the reality of another. Is that really anyone’s business?
That’s exactly my issue with the “mh” world–they want to challenge insistently, relentlessly, any perspective that does not ring true for them. And to my mind, that’s an extremely limited perspective (e.g., DSM). Nothing to explore there, it’s pretty black & white, which I think is a completely unrealistic read on anyone. Everyone is complex, and how they embody that is their business.
But if you don’t believe in psychological injury, then perhaps you will not recognize when it is happening? That is where I go with that. At least in my reality, that would be the case. Not yours, of course, because there is no such thing. But I would wonder about my psychological safety in that case, is all I’m saying.
Report comment
It is like “growth” for most of us after the age of 25, and there are educationalists who go there, and say that such “growth” never ends, in other words, I hear what you’re saying, but I’m also noticing that most of the “giants” they would be referring to are strangely small, and hardly beyond human dimensions.
Report comment
“It is like ‘growth’ for most of us after the age of 25”
This I understand and for the most part I agree with it. Perspective and good defenses are part of maturing.
Still, in a systemically oppressive community, psychological trauma (as I would define and perceive it) can be a way of life, and we don’t even notice it. Marginalization and the discrimination that occurs from this is chronic systemic and social trauma, most often unrecognized. But the effects of it are more than evident, and it is felt profoundly. I’d like to shine a big ol’ light on that.
And where there is marginalization and discrimination, there are marginalizers and discriminators. I’d like to shine an even bigger light on that–what I’d call ‘the shadow’ of our society. The gaslighters, bullies, manipulative liars, etc.–the ones who pass it along freely, poisoning others’ minds for their own gain OR protection, without a second thought…
Report comment
The “disease model” of mental distress better describes the medical model because it is a more basic description of their position. The disease model describes mental distress as a disease whereas the medical model implies that it is a disease by describing it as a medical subject.
Report comment
Yeah but see what I mean? You’re replacing “mental illness” with “mental distress.” I used to do the same thing, except using “emotional distress.” Thing is, only a certain portion of those labeled “mentally ill” are experiencing distress. Some are causing others distress. And some are exhibiting behavior that others find disturbing and confusing but they are fine with themselves. All these plus even more disparate personal circumstances are lumped together as varieties of “mental illness.” It’s like putting broken glass, hypodermic needles and jellyfish into a scientific category because you can hurt yourself stepping on them at the beach.
Report comment
I absolutely love the way you put things oldhead! So relatable, so true!
Report comment
My biggest “distress” came in discovering that I was going to be committed to a state hospital, that is, detained and stripped of my liberty. Nobody had an adequate answer for that kind of “distress” whom I encountered. Nobody had an answer for that kind of “distress” that didn’t wind up increasing it. I spent time in a psychiatric prison, and I was very relieved, of “distress” and so forth, when I was eventually ‘discharged’ from it. Tight spots increase “distress”, but I could definitely do without that kind of “treatment”, should it’s tacit reason be for the relief of “distress” or what have you.
Report comment
I believe that the term “medical model” has evolved over time. Before 1980, the medical model referred to understanding “psychoses” (of Freudian Theory) as medical problems; afterwards, the medical model expanded to refer to all mental distress. The “disease model” and “medical model” refer to the same thing; is the “disease model” a better term?’
Report comment
No, again, unless you first define what you’re talking about models OF.
Anything “treated” by a shrink is a medical model approach.
Report comment
I am describing models of social welfare problems of “mental distress” (emotional distress or emotional suffering in reaction to distressful experiences) and “anti-social” reactions to the distress. I am referring to social welfare problems rather than medical problems. I believe there is enough census for definitions. Although, you are correct that there is nothing “there,” society regularly discusses abstract behavior patterns.
Report comment
“Society” is also an abstraction. 🙂
Report comment
I think you may be right, Steve. The psychiatric theology seems to describe a model of social welfare problems.
For example, child abuse is a social welfare issue. And covering up child abuse, so most people in society don’t hear about our society’s lack of protection for children, does seem to be the number one function of the psychiatric industry today.
This is why there are so many foster children drugged by psychiatrists. This is why “the prevalence of childhood trauma exposure within borderline personality disorder patients has been evidenced to be as high as 92% (Yen et al., 2002). Within individuals diagnosed with psychotic or affective disorders, it reaches 82% (Larsson et al., 2012).”
The psychiatrists offer society a quiet, albeit un-Constitutional, way to cover up embarrassing societal problems.
Report comment
A quote from Richard Nixon, “If a doctor does it, that means it’s medicine.”
Maybe we should keep doctors away from everything emotional.
Report comment
Thank you once again Robert Whitaker for speaking the truth about psychiatric drugs. My son went through a 2 week psychosis over two years ago with very minimal meds (benzos) for a few days just for sleep. Using an Open Dialogue informed approach, he came through the experience using a home respite with family with him and trying to connect 24/7. Since then, he has not experienced a recurrence. He continues to work through some issues, pain and existential challenges related to growing up with his therapist who is also a mindfulness teacher. I cannot say enough positive things about the power of simple meditation and living in the present. He and several of us have started meditating and found it very helpful with anxiety, worry and mood regulation.
All of this together has kept us moving forward individually and as a family with minimal use of meds. Long term meds are NOT necessary for recovery from all experiences of psychoses and other extreme experiences and overuse may actually interfere with the insights that may be gained with dialogue and mindfulness practices. I say this as a parent, a peer and as a clinician. Each of our expertise counts and we all have the right and the duty to examine the research and weigh in on the use and overuse of medications in psychiatry.
Report comment
Hi Truth in Psychiatry,
Whenever I read your wonderful story, I worry that psychiatrists like Pies will dismiss your input by saying that your son had a “brief psychotic disorder” and that people with brief psychotic disorder do recover quickly, and that psychiatrists see this happening frequently after standard ‘best practice’ in the hospital.
Indeed when my loved one was in the hospital and put on antipsychotics, we were told that being on antipsychotics would ‘hopefully just be for a couple of months’. The problem was that for our loved one, milder symptoms morphed into much more serious symptoms first after forced commitment, (what kind of logic is behind separating people from the people they know and trust at the time when they are experiencing troubling thoughts and confusion over reality!), and then again after drugs were introduced , withdrawn and changed. Of course the ‘narrative’ of psychiatry insist that all worsening symptoms are always due to ‘illness’ not ‘drugs’.
I wish so much we had been able to give our loved one the type of support that you gave your son –and wonder if our story would be more similar to your story if we had.
This is one of those many areas that psychiatry refuses to consider – does their “treatment’ (immediate use of antipsychotics, lack of ‘wrap around’ support from loved and trusted people etc.) change the trajectory of some people from having ‘a brief psychotic disorder’ (to use psychiatric terms) to something more severe and chronic. If psychiatrists believe in brief psychotic disorder (and they do) why on earth does their ‘best practice’ not, AT THE LEAST, include a ‘wait and see’ period of at least 6 months, before subjecting people to drugs which they know have such huge effects, when they themselves know many may recover quickly without them,
This would all be figured out if ‘ethical’ studies for first episode psychosis, such as the one Robert Whitaker describes in his response below, (and similar to your son’s experience) were undertaken.
Report comment
Hi Bob. Thank you for this excellent article! You will not receive a direct response from Ronald Pies, which is unfortunate as I’d enjoy seeing him attempt to engage with your strong data-based argument, as well as the serious ethical issues you raised regarding these two studies. I don’t understand how either of those “quality of life” studies could have been approved by a non-corrupt institutional review board.
You’ve covered a lot of territory in your response and have done a nice job of critically analyzing the relevant science and justifying why it is credible and important for a journalist like you to analyze it. But you didn’t address an issue that I suspect Pies would say is critical, so much so that it justifies his simply dismissing anything you say without the need to even consider it. I’m speaking of the view that practicing clinicians are uniquely qualified to understand the nature of “clinical reality.” According to this view, clinicians have access to a uniquely valuable source of information that people like you don’t – clinical judgment and experience – and as a result, they better understand the nature of “mental illness,” the effectiveness of psychiatric drugs, and how to interpret the meaning of scientific articles. No amount of thoughtful critical analysis by a person like you will ever be compelling enough to be taken seriously by someone like Pies, because you don’t understand what he has seen (or thinks he has seen) with his own eyes in the clinic.
A quick anecdote: In 2004, as a Mayo Clinic postdoc, I was able to arrange for Irving Kirsch to present a grand rounds talk to the Department of Psychiatry and Psychology. His talk was titled, “Antidepressant medications: The emperor’s new drugs.” You can guess how popular it was with the psychiatrists in attendance. The next week’s speaker was a psychiatrist who studied the biological aspects of depression. In introducing the speaker, the department chair (a psychiatrist) noted that given his extensive clinical experience with depressed patients, the speaker was in a better position that Kirsch to understand the “true effectiveness” of antidepressant medications.
The same thing happened to me when I debated the president of the European Psychiatric Association on the efficacy of antidepressants (http://www.madinamerica.com/2013/09/united-states-biomedical-model-five-anecdotes/). My opponent, a “scientist” with 600+ publications, invoked his clinical experience to trump findings from the clinical trials literature on antidepressants.
Bob, you and I encountered the same problem: when faced with contradictory evidence from well-conducted scientific studies, some psychiatrists invoke clinical experience to dismiss it. As I see it, the issue for Pies isn’t so much that you have mischaracterized the nature of clinical reality, as it is that you are not qualified to comment on it because you are not a clinician. I doubt he will take your critical analysis seriously, compelling as it is, because you are a non-clinician and thus do not have access to his privileged reality.
In my view, the best response to Pies is to vigorously critique the accuracy of clinical judgment. That is breathtakingly easy to do. There is a massive literature on the fallibility of clinical judgment and the superior accuracy of controlled scientific research (at least when it’s not being manipulated by pharma or to promote guild interests). Clinicians are lousy at judging the effectiveness of drugs and psychotherapies, and the nature of psychological problems, through subjective observation. Reasons for this are obvious (e.g., clinicians see an unrepresentative sample of clients, don’t receive accurate feedback, don’t see people who improved without treatment, don’t know what would have happened if their clients received a different treatment, etc.). “Clinical experience” led psychiatrists to believe that lobotomy and insulin coma therapy were safe and high effective therapies, led physicians to believe that leeches and bloodletting were effective, and led psychotherapists to believe that recovered memory techniques were helpful. An excellent, recent summary of why clinical judgment is often wrong can be found here: http://pps.sagepub.com/content/9/4/355.short (brief blog: https://digest.bps.org.uk/2014/07/28/the-mistakes-that-lead-therapists-to-infer-psychotherapy-was-effective-when-it-wasnt/).
A truly scientifically-minded clinician would never invoke clinical experience to trump scientific research, and would understand that one should be embarrassed for doing so. Yet Pies prides himself in smugly dismissing you in favor of his clinical experience. I submit that if you wish to do real damage to Pies, call him out for engaging in the kind of shoddy clinical reasoning that first-year psychology students know to be fallacious.
Cheers,
Brett
Report comment
Excellent points Brett. What I find so interesting about Pies is his ABSOLUTE unwillingness to consider the possibility that he might be wrong. It is this that precludes me from taking him seriously either as an experienced clinician or “a man of science”.
Report comment
Robert Whitaker – this is a great comment by Brett and I agree you would do well to emphasize the weakness in Pies’ privileging of clinical experience above data/evidence of other sorts.
Report comment
And Pies implies that only the clinical experience of those who have the power to prescribe counts. And that somehow their clinical experience renders them exclusively able to evaluate studies.
Report comment
This is the path we need to follow but I fear it isn’t available for most people. The cost, the availability, the quality of private therapists is beyond the reach of many, particularly those whose difficulties came about as a result of poverty and disadvantage. The entire system mitigates against those with severe states.
Report comment
Yes, these are excellent points.
In my experience as a patient, my treating psychiatrists also absolutely refused to hear what I was telling them about my extremely negative experience of their drugs.
They INSISTED their clinical experience was that the drugs worked, and therefore, my experience was not valid. I have to wonder how many other patients experienced such behaviour by psychiatrists who then go on to claim that their “clinical experience” is that the drugs work.
If they will only listen to the patients who agree with them, and roundly chastise and/or forcibly drug those who don’t, then their “clinical experience” is worth nothing at all!
Report comment
I am likelier to believe a high school drop-out who consistently tells the truth than some experienced clinician with a doctorate who lies all the time.
Btw, I know Bob has a college education. I was using that analogy to make a point. Regardless of degrees and expertise, known liars are unworthy of our trust.
Report comment
Good point, mik, my experience was that the more you tell a psychiatrist that their drugs are making you sicker, the more drugs they give you.
My husband finally had to tell my psychiatrist that I was “too drugged up” at one point, and take me off some of the drugs himself. It was absurd.
The psychiatrists can’t handle the reality that their poisons are not actually “wonder drugs” for all of humanity.
Report comment
It is difficult for me to find the words to express my feelings on the “Psychiatric Times” article “Quality of Life and the Case for Antipsychotics”
August 10, 2016 Ronald Pies and Joseph Pierre .
Judged as a (supposed) schizophrenic at age 19 , I now am 48 and have resisted taking the poisons the (supposed) doctors call medicine during my adult life.
I think anyone who claims these psychiatric drugs are medicines, the claimant should take the drugs themselves. “The proof of existence must come from those who make the claims.” https://www.logicallyfallacious.com/tools/lp/Bo/LogicalFallacies/145/Proving-Non-Existence
If the doctors protest and say” But I am not ill!” , that is exactly what many of the psychiatric patients claim.
The doctors will only get their freedom after three months in hospital/jail and being drugged each day.
Report comment
There are a lot of interesting comments here. I will try to address four particular questions.
1) What would an ethical study on antipsychotics look like?
I think this is the very question that needs to be discussed. Medicine is in thrall to placebo-controlled double blind studies, but it seems to me, there are limited opportunities to use such studies with people diagnosed with psychotic disorders. But here are my thoughts.
First, you could have placebo-controlled, double blind studies in first episode patients, who were medication naive, as long as there was “environmental care” still provided, meant to keep people safe, and also, in my opinion, you still allowed for an as-needed use of a drug to help people sleep, since lack of sleep can be such a contributing factor. A cochrane review of the literature for such first-episode studies of antipsychotics found only a few, with something like 500 total patients, and in those studies, antipsychotics did not show superior short term efficacy to placebo for knocking down psychotic symptoms.
Beyond that, I think you need studies that basically compare treatment protocols and outcomes based on differing uses of antipsychotics. Harrow’s study is a well-designed longitudinal study, precisely because he does identify different types of patients with different prognoses at baseline, and thus you can see different outcomes according to medication use within those subgroups.
The Soteria experiment is an example of a quasi-randomized study that compares different treatment protocols, and found that a protocol that provided psychotic patients with a home and minimal/selective use of antipsychotics led to superior two-year outcomes than regular care and use of antipsychotics.
The Wunderink study is an example of a the use of randomization to set in motion different treatment protocols, and showed that a tapering effort led to better long-term outcomes.
In short, I think you need such protocol comparisons (which already exist in the literature) to ethically assess antipsychotics (beyond what a naturalistic study like Harrow’s) and the reason is you still need to have an environment of care.
b) To Oldhead: I think what APA adopted in 1980 when it published DSM III was a “disease model” rather than a medical model, but I guess I was using the APA’s terminology in quotes. As to evidence-base and evidence based, the meaning difference is this:The “evidence base,” meaning the collection of scientific evidence, provides a means to practice “evidence-based” medicine, meaning medical practices that are consonant with that scientific evidence. If you have a flawed “evidence base,” or assessment of it, then your “evidence-based” practices are going to be flawed too.
c) What about the outcomes for the people who do well during the placebo washout period. This may surprise many, but this is designed to help suppress the placebo response in the trial, which the FDA approves of. And of course, if you wanted to truly assess what happened to the group of patients who entered into this study, you would want to follow these patients too.
d) to Brett Deacon: I should have made this more clear, because as you say, Pies and Pierre are asserting that their clinical experience is necessary to know what is best for their patients, and scientific evidence only goes so far. Well, the history of medicine is filled with examples of doctors (and especially psychiatrists) adopting therapies and seeing them as beneficial that were later seen as harmful. That is one of the main themes of Mad in America. I suppose lobotomy may be held up as the prime example of that, as it was touted as a miracle brain surgery for the longest time, and now is viewed as a mutilating surgery. The whole point of “evidence based” medicine is that begins with the understanding that clinicians can be deluded about the merits of their therapies (they are bound to see them as helpful), and thus the need to be guided by scientific assessments of those therapies. I should have made this clearer.
Report comment
If you have a flawed “evidence base,” or assessment of it, then your “evidence-based” practices are going to be flawed too.
My point exactly. Who determines what is considered “evidence” and what is considered groundless speculation? And it seems that for whatever they do to be considered “evidence-based,” all they need to do is dig up something from a Torrey/Jaffee propaganda sheet and use it as an “evidence based” justification for forced drugging. “Evidence” is used to argue both sides of any debate; the same evidence can be used to argue opposite positions.
Report comment
Further, wouldn’t one normally assume that any medical/scientific determination is based on some sort of evidence that it is correct? Why is “evidence based medicine” being heralded like it’s a new breakthrough in medical science?
Report comment
Because its been ‘clinically proven’ that 4 out of 3 patients don’t look beyond the words and examine what they actually mean? Trust me, I’m a Doctor lol
Report comment
4 out of 3 is a pretty decent percentage!
Report comment
It is oldhead 🙂 “Clinically proven” was one the Gruen team had a bit of a look at. Some of the advertising that is being used with certain products and how they can make such outrageous claims about them.
Seems an important point from the masters of spin:
The Gruen Transfer …. is the moment when consumers enter a shopping mall and, surrounded by an intentionally confusing layout, lose track of their original intentions, making consumers more susceptible to make impulse buys.
Consider that in the context of ‘consumers’ entering the shopping mall of psychiatry?
https://en.wikipedia.org/wiki/Gruen_transfer
Report comment
My point being that what Mr Whitaker is saying about “Evidence Based Medicine” and the actual “Evidence Base” is consistent with the con.
Report comment
Writing as some one who has a history of mental illness, if a group of psychotic people you describe above can be found for a study (impossible), I would want a study of antipsychotic VS benzodiazepine (like Atavan) to reduce symmptoms.
_________
In regular medicine the medicinal drug is applied with reason, if these certain conditions exist , then apply this amount of drug.
If no psychotic symptoms are being exhibited then there is no reason to apply “this amount of drug”.
This is the insane bit from Psychcentral.
““How long is medication needed to treat schizophrenia?” The answer is usually: people most benefit from taking medication for schizophrenia most of their lives.”
http://psychcentral.com/lib/schizophrenia-the-challenges-of-taking-medication/
When they do not know if the returning psychotic symptoms are from the drug withdrawal or the pre-existing mental illness.
Report comment
Another example of Pies’ muddy thinking where he touts, and produces, `scientific evidence’ to support his case then, in almost the same paragraph tells us that it is experience and observation that is the key. He so often tries to play all hands at once but seems incapable of seeing it.
Re LOBOTOMY “- psychosurgery is back with a new name—neurosurgery for mental disorders—and with renewed confidence in its benefits.” (now Cingulotomy and capsulotomy or even more respectable, “small lesions”)
Two technologies are now available that produce small lesions in the brain:
1. Stereotactic microablation (late 1960s)
2. The gamma knife (no burr holes necessary) irradiates small intracranial targets (brain tissue) with gamma ray photons. Instead of burning or freezing brain particles, gamma radiation deranges molecules in the target cells so that they can no longer survive.
!!!! This, plus the ongoing and increasing PR campaign to change the image of ECT might be a worrying consequence of discrediting the drugs. They’ve got to have SOME miracles, it seems.
Report comment
Psychiatry will say anything – and do *anything* to its patients/victims – in order to preserve its illegitimate status as a medical specialty. This has been its history and this will be its future.
Report comment
I hope I am not not wandering off here.
IN my opinion the basis of any ethical study would have to be one of honesty.
I think the underlying blunt disagreement is whether the chemical approach is a solution or not.
From my perspective I believe the Talking Treatments are the solution as they can be proved to return a person to full longterm recovery and independence.
The Talking Treatments would work for “schizophrenia” in the same way as they work for anyone else – there’s no great difference.
The Link below (for me) would represent the basic principles behind the Talking Treatments:-
http://time.com/4470517/neuroscience-of-mindfulness/
I don’t know much about the different sides if the brain though.
Report comment
I don’t think there can be any comparison between Full Longterm Recovery through “Psychotherapy” (which I know can be achieved) – and long term chemical disability which the present day Psychiatric model offers.
Report comment
It looks to me as though everyone is painfully confused. I think the only reliable sources for “expertise” are those who have gone through this. There is wisdom in healing.
Report comment
Alex,
A few months ago I listened to a chair in one of my 12 Step Groups given by a well turned out young person in their mid twenties.
They said that originally they had a variety of young person issues and were eventually prescribed medication.
They said that the medication was causing problems so the doctors increased the dosage and went on from there to introduce a diagnosis of schizophrenia. Eventually things got so bad that they lost the power of speech and basic performance. It even got to the stage that their parents were preparing for life long institutionalisation.
They said that they then tapered off the drugs with help, regained functioning and got completely back into swing of life again.
This is probably the typical experience – up to the point of TAPER.
Report comment
I can relate to that story, except in my version, they called it “bipolar,” which turns out was never the case, I can see that now. I actually didn’t argue at the time, I felt relieved that it had a name and their were drugs to take for it. In reality, I thought it was kind of interesting, I didn’t at all feel a stigma from it at that time, why would I? For me, it did not translate into “disabled” back then, so I went with it, and then went to work and to live my life. It wasn’t until 19 years later it all caved in. I’d no idea that these drugs were so insidiously toxic, it all just snuck up on me, and then WHAM! That’s when the really bad stigma and toxic system abuse began for me.
Wading through and navigating all of that, after withdrawing, is what completed the journey I had begun 32 years ago, which got drugged up big time. Finally, off the drugs, I was able to continue my path of evolution, whew. It’s very relieving, like, suddenly, there is ease, which had been sorely missing in my life–thanks to the drugs, which made everything way more effort than it needed to be. I also have clarity now, not possible on the drugs. They inhibit neural flexibility, which is not at all healthful nor natural, to my mind, to be rigid in thought.
Although at the time I was diagnosed, I see what they went by, but I also see what they did not weigh in, which was, of course, who I am and how I process. they forgot to listen to my voice. They only heard their own voices, telling them fibs, based on misguided information, which is programmed in their neural pathways.
When we have that kind of education and practice applying it over and over again, it becomes a program, and that’s why “mental health” clinicians, on the whole (from my experience) don’t recognize PEOPLE as who they are, but merely as a projection of their own shadow. Not always, but terribly often. Especially their clients, because that’s their bread & butter and they are vulnerable.
But I imagine it’s not limited to that. In fact, I know it’s not. I know how therapists talk about people, I was around a bunch of them in graduate school. They gossip in diagnostic terms, it’s pretty interesting to witness, if not a bit disturbing.
So you’re saying that after tapering, this is where experience really become diverse? Some heal and get on with life, and others don’t? I just want to be clear about whether or not this is what you’re saying, because if it is, I agree, and it puzzles me. Why do some people seem to heal and others don’t? Or perhaps the time for healing varies? I don’t know. But I think it’s a very important question to ask. I’ve definitely noticed a fork in the road here and have wondered how this would be explained. I’m sure there are a lot of avenues of exploration, here.
Report comment
Alex,
In 12 Step Groups most people have some type of gruesome past and this is why they attend. I related this story as an example of how easy it is to get trapped in the medical system.
I think some people heal and these are the people that recognise the medical fraud but most don’t heal – they get trapped in the system.
Report comment
Yes, I agree, people are caught off guard because they’re not told the truth and they go deeper into what they feel they can trust, mainly from a desire to get well and feel good again– and wham, suddenly there is betrayal and deceit all over the place, all this re-traumatization from trying to get help and support.
Still, I’m wondering how some people get out while others do not/cannot. Some people with horribly traumatic backgrounds who end up getting caught up in the system get out of all that, heal from all of it, and move on with their lives–I’ve seen many examples on here alone, over the years, and I imagine we’re all over the place–while others get stuck. I’m curious what the difference would be between those who make it and those who don’t?
I don’t at all believe that the worse the trauma the more likely one is to not heal and find freedom, not in the slightest. And while I think money can be a factor in this, I also don’t think it’s definitive. I had no money and I was able to get out and heal, thanks to all the new stuff I was learning, to which the desire to heal led me. I just happened to find what I needed, I left no stone unturned in my quest for healing and clarity.
So to my mind, there is something even deeper going on which distinguishes the fate of people.
In fact, I think the trend is that the more profound the trauma and the deeper the entanglement with the system and all that, the greater the potential for going in the opposite direction. The worse the trauma, the greater possibility of personal growth and evolution–by leaps and bounds–and acquiring wisdom. We can grow so much from life’s trials and tribulations, and many people do. Why do some not?
That would be my research question because I think there is a lot of subtle information here that is of value in understanding what it means to be human.
Report comment
Alex,
I think when people suffer they can become ‘ruthlesslessy’ focused on ‘recovery’ , especially if they get a little taste of it.
I was genuinely spell bound in my early 20s by the psychiatric approach but once I realised that there was something I could do to pull myself out I focused on it.
As the years went by I had forgotten about my experience – until one day I discovered that my GP surgery had my name on a Severe Mental Illness Register and were gaslighting my records.
Report comment
“As the years went by I had forgotten about my experience – until one day I discovered that my GP surgery had my name on a Severe Mental Illness Register and were gaslighting my records.”
Wow, Fiachra, that is scary, to be on “a list” like that–that there even IS a list like that! Coming out of all this is just one rude awakening after another, isn’t it? Damn.
Report comment
Very dodgy, Alex.
My name was placed on this Register in 2002 when I was working as a subcontractor in the House of Commons Westminster and physical tests were carried out on me through deception.
When I found out the doctor surgery were apologetic at first – and then turned nasty.
In 30 years I’ve been in the UK I have been about my business and have never taken a penny in sick pay.
(I recovered in 1984 as a result of stopping treatment).
Report comment
Understanding the laws of detachment were the key for me.
When I stopped the drugs my head was full of anxiety – but I was okay when I was able to detach.
Report comment
Alex – I found reading about your story and then your questions about why some people heal and others get trapped very interesting. This is the question most prominent on my mind as I try to support my loved one on the journey back to wellness.
Report comment
Sa, I know in my case, the role of my partner in all of this was significant, although it wasn’t as simple as his supporting me through it all. He was as caught off guard by all of this as I was, and, both, emotionally and intellectually, he was much less prepared for life’s challenges than I was. I was way more philosophical about things, and neutral, just by nature. I’m also a problem-solver, I’ve always been that way.
Although during this time, that was not the case, as the psych drugs withdrawal had me wringing my hands–and brain–quite a bit. Took a couple of years for that fog to clear altogether and feel my intuition again, as well as to regain my self-perception. My emotions had to regulate, also. A lot had to come back into balance and regenerate after the effects of the drugs, and that took some time.
The challenge for us was that my health crisis due to the withdrawal from drugs triggered his unresolved issues, so we both ended up going through our respective journeys at the same time, and it affected us each in different ways, which put us at odds, we were power-struggling all the time. I spearheaded the healing, because I was the one who had been disabled, and in addition, it was me who was training to be a healer, this was all my gig from the get-go.
In our case, it was our relationship dynamic which created and then embedded the crisis, we each played a role. He finally took my cue and example and followed the healing path I was on, he attended the healing school I went to, when he saw how I was getting such great results from this program. This put us on the same page, with better clarity and perspective, each of us owning our part in all this.
Our entire way of life changed, and eventually, so did our relationship roles. I’m his teacher now, we got it straight. We’re not stuck anymore, as we had once felt, and learned how to create together in the most efficient and fun-loving way now. We did a 180, thanks to all this. The relationship is what needed healing, not just us, individually.
Aside from playing out the healing this way, in partnership, I think another reason that I was able to free myself from all of this is simply because I did not have a vision of myself as was being projected onto me, all that stigma and false conclusions about who I was as a person, from superficial data. I knew full well this wasn’t me, but It was seriously hard dealing with all that while I had no defenses of which to speak, given that my mind and emotions had been temporarily tattered by the drugs. That was a 1-2 punch.
But that was part of my education, in the end. I learned a lot about people, for better or worse. Woke me up. But that was definitely one of the most challenging aspects of healing–naysayers, stigmatizers, and marginalizers, all along the way. Learning what this was all about taught me more than I ever wanted to know about our society, but it sure did explain a lot. And it’s why I’m here.
Healing requires a positive, self-affirming, loving, and grounded environment. We can do it alone or with others, as long as the above requirement is met. At least, that’s my perspective.
Report comment
Hi Alex – no room to respond under your response to my comment but just wanted to say thanks -lots of helpful info!
Report comment
Fiachra,
Did the person you describe talk about how long they experienced loss of basis functioning etc., and then how long it took after the taper to return to normal functioning?
Report comment
Sa,
No, they didn’t mention how long they were sevetely incapacitated for. But they did mention that it took more than a year or to regain very basic functioning.
You might have seen this particular example before from (one of) the founders of the cepuk.org (he experienced quite a hard time):-
https://www.google.co.uk/url?sa=t&source=web&rct=j&url=https://beyondmeds.com/2015/07/18/antidepressants-ruined-my-life/&ved=0ahUKEwiJ0PH_tojPAhUI3CwKHfSLD78QFggbMAA&usg=AFQjCNHAh9_rel_cu41srEw7Ir-BBkmnKg&sig2=s6e5wNeetIIT8cIxKpt6xQ
Report comment
Thanks!
Report comment
Mr. Whitaker, you’ve saved my life and the lives of millions of other Mad people with your accessible, reliable, and empathic writing. These quacks are just envious because you’re talented, you’ve enjoyed a long and prestigious career, and you’re SO much beloved by the public. DON’T BELIEVE THEIR LIES ABOUT YOU!
Report comment
Remember if you are in the cross hairs you are worth something.
Report comment
First they laugh at you, then they….etc etc
Looks like we’re in the fight you stage.
Good work!
Report comment
I hate to always be such a downer, but psychiatry and it’s drug pushing is by far more powerful than ever. Tim Murphy’s bill even passed the senate with only one dissenting vote. It wont be long before family doctors / general practitioners are forced to screen their patients for depression. Schools are already doing such screenings for children and in many area’s forcing parents to comply with both the screening and the treatments that follow, with the power of child protective services at their disposal. This is on the verge of becoming a federal mandate.
Meanwhile, psych drug prescribing is continuing to climb steadily, every year being more than the last.
It’s depressing to think about, but this side of the fence is losing horribly.
Report comment
Eventually direct action may be the only option. I, in my old age, begin to feel the despair and rage that I suspect leads to the storming of the barricades. How long will it be before a movement takes to the streets?
Report comment
Some of us would like to inject reason into the public debate. Dr. Pies is unlikely ever to acknowledge that his career has veered off onto a track that is lucrative and prestigious but will come to a dead end.
Fortunately, most people are more open to persuasion than the comparative few who have staked their careers on the 21st Century equivalent of snake oil. (Clinical experience with snake oil, by the way, was excellent. Its purveyors may have been as sincere as Dr. Pies. Consider venipuncture, too. Bleeding the sick, draining the bad blood, was a prime treatment for most ailments for at least 2,000 years. The clinical experiences of its host of practitioners validated its efficacy. Most patients recovered. Those who died–George Washington, for example–were unlikely to complain.)
The challenge for those of us who want to reduce the harm from “antipsychotics” and other ineffective practices is to reach much larger audiences as well as small but powerful groups such as legislators.
I suggest that those who would take action study the tobacco wars, the efforts to get lead removed from paint and gasoline, and other successful campaigns to curb profitable but harmful businesses. MadinAmerica readers who are interested in action are invited to contact me: [email protected].
Report comment
The challenge for those of us who want to reduce the harm from “antipsychotics” and other ineffective practices is to reach much larger audiences as well as small but powerful groups such as legislators.
True in general. However this week and next at least the specific focus of action should be for everyone to call both their senators and demand they vote against the Murphy Bill (SB 2680), and especially any efforts to attach an Assisted Outpatient Treatment (forced drugging) amendment to the bill. Go to the Organizing Forum for further discussion.
Report comment
Dr Opton, those who died after venipuncture not only can’t complain but clearly died from the illness not the treatment, which is why it continued for so long. I’m sure that it was the colic that killed the babies who died after receiving opium as a treatment for it, and how sad it was about all those deadly illnesses that people succumbed to even when treated with mercury.
Report comment
They feel threatened by you! And they should, they are wrong. You always remain civil and engage in dialogue and meticulous research as much as it is needed, and that is why we are all here, to read it because we know you are being ethical. Keep doing your thing. They are reading what you write and like someone else commented them sneering at you is a high compliment to the integrity of your journalism. Psychiatry is a cult and more and more people are reading what you say too. I fear these psychiatrists are too steeped in their ego and even narcissism to ever see the truth about their “profession” but this web site and leaving psychiatry has saved my life and made it worth living again.
Report comment
Strangely this all just flies in the face of the principles of Evidence Based medicine, which is medical practice based on the judicious appraisal of the best available evidence. It never say’s who should be doing the appraising and seems to be quite clear that medical experience and anecdotal evidence are not substitutes for EBM.
I also pointed out in a post about a similar matter that occured in Finland, how Doctors who go round saying that only they are qualified to appraise the evidence seem very willing to accept evidence from non qualified, zero clinical experienced Drug reps and other industry bosses.
So there you have hit, Shut up Dr Pies and Dr Pierre!!
Report comment
“…how Doctors who go round saying that only they are qualified to appraise the evidence seem very willing to accept evidence from non qualified, zero clinical experienced Drug reps and other industry bosses.”
Nicely said!
And drug reps I have known came out of two-year marketing diplomas. And, yes, they’re delivering critical information to MDs.
Report comment
bob- just want to say again- you are my hero.
on behalf of ALL of humanity, thank you and bless you.
-erin
Report comment
What are the chances that an ethical study of antipsychotics will ever take place?
Report comment
In November 2011 I was admitted to a highly regarded psychiatric hospital for severe psychosis. The doc took a look at me and took me off Wellbutrin (known to cause psychosis) and put me on lexapro and seroquel. Almost five years later I am still on an antipsychotic and probably will be for life. Any reduction to my Haldol results in severe psychosis.
I suppose I would have been a good candidate for an ethical study. I was going through a first episode of severe psychosis. Where would I be now if I had been given a placebo instead of going down the polypharmacy/antipsychotic path? Answer: probably a lot better off.
How can we recruit patients that fit the medication naive profile?
Report comment
As I see it, Psychiatry, Drugs, and Psychotherapy are all based on the idea that people don’t need to feel their feelings. And lots of other stuff works this way too.
Need to find ways to organize and fight this premise.
Nomadic
Report comment
Sadly Dr Pies, a leader in `thought’ in the profession, appears to have a talent for self delusion and an untidy mind. I read many of his articles in the Psychiatric Times and often comment. In a similar earlier support for Antipsychotics piece, Dr Pies claimed (Feb 2016) `Recently, the blogosphere has been buzzing with controversy regarding the use of maintenance antipsychotic treatment.’ and goes on to say, `I would argue that interpreting these complex studies requires an in-depth understanding of medical research design, psychopharmacology, and the numerous confounds that can affect treatment outcome. Unfortunately, A LACK OF MEDICAL TRAINING has not stopped a few critics from confidently charging that psychiatrists are harming their patients by prescribing long-term AP treatment.’
My response was, `Surely you’re not suggesting, Dr Pies, that people without medical training do not have an understanding of research design, psychopharmacology and confounds. Research design is a major part and dare I say, proportionately greater percentage of many disciplines other than traditional medicine… there is a large group of highly scientifically trained researchers who might be surprised to be told that their observations are inadequate because they have not qualified as medical doctors. I’m not sure but appears to be a tiny whiff of a suggestion that there is a conspiracy by these inadequate critics to undermine psychiatry?” Of course he ignores that there is araft of work coming from doctors and psychiatrists that expose all the neuroleptics.
His writing is littered with contradictions, unfounded assertions and poor research and he sets himself up to be knocked off all the time, BUT he is highly influential in that he has The Psychiatric Times as a personal forum and it is very popular among his colleagues, many of whom appear to have similar problems with criticism and clear thinking.
It is essential that this man and other muddy thinking psychiatric leaders with their shoddy/fraudulent “research” be exposed for the pseudoscience it is, continuously, without respite, by everyone, including `renegade’ journalists. Thank you Robert!
Report comment
The set of recent blogs and commentary on this site is simply astounding – not that that’s so new! The rapid emergence within a short forty years or so of a multi-billion dollar pharmaceutical/psychiatry behemoth is truly mind-boggling. The financial and professional benefits accruing to the participating players have been phenomenal. But, just as in cases like the Viet Nam War or our current embroilment in the Mid-East conflicts, or the tobacco effects scandal, or climate change… the official story of what’s going on, the merits and demerits of our commitments and practices, despite ongoing and accumulating evidence to the contrary, establishment voices continually assert and attempt to justify the official story line with neither logic nor evidence that is persuasively compelling.
The voices in support of the psychiatric status quo claim professional and scientific merit and credibility. Sometimes they appear to rely even more on their humane and empathic response to the patients they serve in support of their claims. The latter is problematic in two respects: 1) It is contrary to the grounds for being objective and impartial in their thinking and conclusions regarding critical questions about psychiatry and 2) it is laughably ironic if their claims are meant to characterize the general practice of modern day psychiatry. That practice is predominantly 1) “diagnosis” (at best a medical metaphor, but possibly necessary to get paid) as per the DSM definitions and 2) drug prescription. You can see two to four patients an hour doing that which pays much better than any time consuming therapeutic counseling. Anyone who has observed the actual practice of most psychiatrists can appreciate the truth of these claims.
I am a retired PhD Social Psychologist researcher and former small business owner. I have had a variety of involvement with issues of mental illness/health. I dispensed some of the first Thorazine pills in the early 1950s to patients as a psychiatric aide at the Menninger Foundation Clinic in Topeka, Kansas, then the Mecca of Freudian psychoanalysis. I have also conducted research related to mental health. I have not had the misfortune of being a psychiatric patient. However, I have two friends with adult children in their late 40s who had serious ‘psychiatric issues’ at college age. Both are now on ‘permanent’ disability, both on psychiatric meds, one relatively stable, the other periodically quite unstable with meds frequently being ‘adjusted’. The father of the first is a medical research scientist of some renown. We often discuss science related issues. When Whitaker’s “Anatomy of an Epidemic” was published, I loaned him my copy and suggested that we might discuss it. Well, the next time we met he was angry: “Whitaker isn’t a scientist! He’s a journalist! Why should I believe anything he says?” End of discussion. When I raised some of the concerns of the book with the Mother of the other son, hoping for some discussion, she was not at all open to it. The ‘medical profession’ has spoken definitively on the matter and she wasn’t about to ‘rock the boat’. In both cases, it seemed that the original trauma these parents experienced in the breakdown their sons went through at college age was still a very sensitive issue. Anything that might risk a recurrence could not even be contemplated.
I have another friend of nearly twenty years, now in her late 50s, who is also on disability – I’m her Social Security ‘payee’. At age 14 she had most of her spine fused due to severe scoliosis. She graduated from college and was gainfully employed as a software analyst. Those spinal fusions are no longer performed because they lead eventually to ‘flat back syndrome’, accompanied by severe and chronic pain. For years now she has been in and out of numerous hospitals, group homes, motels, jails… Despite my efforts with mental health providers, there has never been an ‘emergency intervention plan’ in place to respond effectively and humanely to her periodic breakdowns. Over and over, it’s been a ‘911 call’, police intervention, and a landing in jail or an emergency room. I’ll comment on just one striking pattern (there are many others): Her ‘breakdowns’ are universally met with “Oh, she went off her meds!” by mental health personnel. Though I have often been in a position to know that the sequence of events was the opposite: She collapsed and then ‘went off her meds’. I could never get anyone to take my assertion seriously. (So much for the ‘caring, humane’ response of mental health professionals.) This is an example of how the prevailing psychiatric mentality leads to easy and false conclusions.
My congratulations to Robert Whitaker and others who are persistently pursuing truth regarding the nature of mental health and illness and are challenging the currently prevailing mental health services system! Keep up the good work!
Report comment
Hi Bob, great article. I took the leap of signing up for Psychiatric Times and publishing this comment on their article (hope it stays up):
Greetings Ron,
First, disclosure: no one put me up to writing this commentary.
I believe the “journalist critics” you’re referring to are Robert Whitaker, who has written several books critical of psychiatry and who publishes on http://www.madinamerica.com. If that’s so—and please correct me if I’m wrong—your conclusion that these critics haven’t “spent a single hour with a grieving or distraught family member, who has seen a loved one decompensate into a life-threatening psychotic relapse, after stopping medication” is inaccurate. I’m wondering: did you ask him or other “journalist critics” about this assumption first?
I’m glad you acknowledge that people other than psychiatrists should weigh in to help “narrate recovery stories”. Here you are, verbatim: “We would add that, in addition to families, mental health workers of all kinds – counselors, social workers, nurses, and psychologists to name a few – share a privileged position from which to observe and facilitate the recoveries of our patients. All of our voices—not just those of psychiatrists–are vital in helping to narrate those recovery stories.”
What I notice missing from your list are peer supporters who have lived experience and work with others going through hard times, and also people who have recovered (or not) and who aren’t “mental health workers”. I’m curious, why? It seems to me that the people who have actually gone through the experiences that land one in psychiatric treatment would be the most valuable group to dictate the terms of “recovery stories”.
I was in psychiatric treatment for four years for so-called major mental illnesses, and I’ve been out of that treatment for 9 years, during which time my life has significantly improved due to many intersecting factors, one of which has been my rejection of the recovery narratives that psychiatry propagates. Under your terms for what qualifies as having a voice in whether the antipsychotic drugs are effective or not, thankfully I’m in: I indeed spent 8 years in human services (and now 3 as an educator in the mental health world) and a lot of hours with persons, families, friends, and strangers who are having bewildering experiences, sometimes predicated by having just withdrawn from psychiatric drugs. For the record, I’ve also swallowed or been shot up with over twenty different brands of these, but again that doesn’t cross the threshold under your rubric. I’m curious, have you tried the psychiatric medicines? I’d say from my firsthand experience as both a “consumer” and a “witness” (that you appear to value), most psychiatric drugs are doing more harm than good.
I also spent a lot of time stealing expensive psychiatric articles from online journals that I accessed by posing as a student at Dartmouth. I spent a year combing these through, first with an interest in what had happened to my “chemical imbalance” since I was no longer taking psychiatric drugs and was feeling fine, and later getting hooked into the the mass cover-ups, biases, collusions, and unethical treatments that Whitaker documents in his writings.
Now, you’ve just published a case for antipsychotics here by citing two studies. And like most of the studies I encountered in my non-professional reading of the scientific literature, these studies are flawed to a point of absurdity. Whitaker provides you a rebuttal here: http://www.madinamerica.com/2016/09/confessions-of-a-trespasser/
I’d like to ask, if indeed Psychiatric Times will publish here my non-conforming comment: will you please address his criticisms of those two studies?
Cheers,
Steven Morgan
Report comment
Thanks Steve. I’ll be curious to what you hear back from Psychiatric Times.
Report comment
Hey Bob,
Here’s their response (on the article page under supplementary statements):
Some Methodological Issues: A Supplementary Statement from Drs. Pierre and Pies:
Our commentary cited six studies of “quality of life” (QOL) and antipsychotics (AP) that involved a placebo control (Hamilton et al, Nasrallah et al, Beasley et al, Leucht et al, Witte et al, and Isitt et al). All these studies found an advantage for AP use vs. placebo, with respect to improving QOL. Like all studies, the ones cited have limitations and methodological shortcomings, and we carefully qualified our conclusions as “provisional”, pending more long-term placebo-controlled studies.
Some critics argue that APs make patients with schizophrenia worse (or even cause “brain damage”), but then simultaneously invoke the speculative notion of “withdrawal” to explain away the results of the studies we have cited, arguing that these studies did not use “true” placebo groups. But if APs actually make patients with schizophrenia worse, then discontinuing the AP ought to make patients better. Conveniently invoking “withdrawal” to explain why, in fact, patients with schizophrenia who are taken off APs do not usually get better is contradictory. (To critics who respond, “Oh, we concede that antipsychotic medications may be helpful in the short term, but they make things worse in the long term,” we would reply that there are no convincing randomized controlled data, either on the basis of relapse frequency or quality of life, that demonstrate that antipsychotics worsen outcome in the long term. As the Sohler et al study concluded; i.e., “Our study did not support the hypothesis that long-term treatment with antipsychotic medication causes harm.” [Am J Orthopsychiatry. December 14, 2015]
Critics also confuse two uses of the term “withdrawal.” One use means, “stopping or discontinuing a drug.” The other refers to withdrawal syndromes, such as the kind experienced when a patient is suddenly taken off a barbiturate or opiate, and experiences shivering, diarrhea, hallucinations, seizures, or even death. There is no such documented “withdrawal” syndrome associated with discontinuation of antipsychotic medications—even sudden discontinuation–except for generally mild and transient cholinergic rebound symptoms, such as hypersalivation, cramps, or diarrhea. These are usually seen within a few days of stopping the AP; are usually mild and self-limited; and rarely need clinical intervention (In rare instances, they are easily managed with anticholinergic medication). These short-term symptoms are quite distinct from a relapse of the illness, which typically occurs 1 or more months after stopping the medication. Thus, the notion that the placebo groups in the studies we cited did worse because they were “in withdrawal” and not “being treated for withdrawal” is completely unsupported by any empirical data.
A “true placebo group” is a group that is given a placebo—period. There is no other definition in the medical literature. It would require an individualized, patient-level analysis in order to demonstrate that any particular patient taking the placebo was “in withdrawal.” We are not aware of any such data. The notion of a “supersensitivity psychosis”, as invoked by some critics, remains only a speculative and theoretical possibility—not, to our knowledge, one confirmed by any biological study (PET, fMRI, etc) of the brains of actual patients.
Finally, while all studies, including the ones we cited, have methodological limitations and potential biases (e.g., pharmaceutical company sponsorship), we have yet to see critics cite a single controlled study, using standard measures of QOL, showing that quality of life improves for patients with schizophrenia when they are no longer taking their antipsychotic medication.
Report comment
Steven,
Was your comment published at all? I couldn’t find it; I may have missed it. What I found instead was a love-in between the author and his “fan base” (his words).
Liz Sydney
Report comment
It is ironic that Pies tried to appear balanced in this way:
“We acknowledge, of course, that psychiatry’s critics often include the voices of dissatisfied individuals who have received psychiatric care. Those accounts are just as important to consider as the many stories of positive outcomes….”
But despite giving lip service to the importance ofacknowledging people who are dissatisfied, Pies and/or his editors proceeded, apparently, to delete the carefully thought out respectful comment by Steven Morgan above.
One can’t really have it both ways. If one’s own site arbitrarily deletes any comments that disagree with one’s position, similar to how a totalitarian state summarily snuffs out any disagreement with the party line, one can’t pretend to be open-minded and be taken seriously.
If Pies came here and respectfully gave his opinion, his comment would stay up even if we disagree. But when other people go there and try to speak, their voices are extinguished. What is Pies afraid of?
Report comment
Psychiatric Times have deleted a couple of my comments too, both involving Pies. Others have also disagreed, very respectfully, and some are permitted to stand, but they are usually academics or one of the very few `dissident’ psychiatrists. One that was deleted involved mentioning the money being made by ECT. This has happened on every site advertising the virtues of ECT – they tolerate dissatisfaction with the procedure but the minute you mention that perhaps there is a major financial benefit and a possible conflict of interest you get shut down. That obviously hits a nerve.
As I mentioned above Pies is nothing if not inconsistent.
Report comment
The comment from Stephen Morgan is there in the comment section of Pie and pierre’s article.
Report comment
Good – it’ll be interesting to see how long it lasts.
Report comment
Ok, you are right – my mistake. I expected it to be near the top of the New Comments, and not seeing it there assumed it was gone – but it is in fact nested at the bottom as a “new” response to an old comment here – http://www.psychiatrictimes.com/schizophrenia/quality-life-and-case-antipsychotics
So it is there now… though will be interesting to see if, as a non-mainstream comment, it is allowed to stay. I doubt that it’s half-life is going to be very long.
Report comment
Back in June Ron wrote this piece -How Antipsychotic Medication May Save Lives
June 01, 2016 | Couch in Crisis, Major Depressive Disorder, Psychopharmacology, Schizophrenia
By Ronald W. Pies, MD
One way anti-psychiatry groups trivialize psychosis and marginalize psychiatry is by emphasizing the adverse effects of antipsychotic medications while denying or minimizing their benefits.1 To be sure, the well-recognized metabolic, neurological, and cardiovascular risks associated with many antipsychotic medications must be taken very seriously. Moreover, antipsychotics (APs) are often used when they are not needed; eg, for the treatment of anxiety disorders2; for “agitation” in nursing home patients; and for “acting out” in adolescent populations. (I spent many years as a psychopharmacology consultant trying to get doctors to reduce their over-reliance on antipsychotics.) On the other hand, there is convincing evidence that in patients with chronic schizophrenia, APs play a crucial role in maintaining remission, averting relapse, improving quality of life, and—importantly—reducing overall mortality.3-5
But even many psychiatrists may not realize that APs reduce the risk of suicide in patients with schizophrenia.To back up a bit: an estimated 20% to 40% of those with schizophrenia attempt6—and 5% complete—suicide7—a risk at least 10 times that of the general public. Suicides are concentrated early in the illness course and are associated with a number of risk factors.
(Here he doesn’t mention being told your future is over, it’s drugs for now and always, with all that implies.)
The only consistent protective factor for suicide was delivery of and adherence to effective treatment.”8 [italics added].
(As usual he hedges his bets with claims of magnanimity then immediately finds reasons to qualify that with the REAL message.)
My response appeared VERY briefly then disappeared leaving the usual gushing praise from his loving disciples. Dissent on David Healy’s blog receives similar treatment. Both seem to love their name in lights.
Here it is now:
“You open with a rather defensive comment about anti-psychiatry. This is really a attempt to demonise your critics and is beneath any honest scientist whose aim should be to welcome criticism as a means of doing better.
Antipsychiatrists? Or observant scientists?
Medication Madness – Peter Breggin 2008
Anatomy of an Epidemic – Robert Whitaker 2011
Pharmageddon – Healy 2012
Depression Delusion Volume One- The myth of Brain Chemical Imbalance – Terry Lynch 2015
The Myth of the Chemical Cure – Joanna Moncrieff 2008
The Bitterest Pills: The Troubling Story of Anti-psychotic drugs – Joanna Moncrieff 2013
The people I’m quoting below, Antipsychiatrists? Possibly, now, after they asked for help from psychiatry, – you’ll never get all the truth from the surveys, the studies, the `literature’ because none of those include the voices of the people most affected.
If anti psychiatry exists as an `evil conspiracy setting out to down psychiatry’, one must ask why? Is there an anti-oncologist movement? They fail many people and the treatments can be diabolical. Is there an anti dermatologist movement? They also fail to help many. Is there an “anti” any other part of the medical profession? Many practitioners are hauled up for corruption, incompetence, and sometimes criminal behaviour, but there is NO “anti”.
Of course none of them can force treatment on their patients so that might be a factor, and most have a fairly firm basis in science as a fall back position which, of course, psychiatry doesn’t.
So let’s look at some of these “anti-psychiatrists”. Perhaps, in hearing their real live voices, you might understand why the dissatification and thereby be able to negotiate some kind of dialogue instead of dismissing dissent, which every authority does at its peril. These things are NEVER one sided.
Some voices:
“They were neuroprotective because they protected you from thought, at the minor cost of getting you addicted and giving you dyskinesia.”
“Any review of the medical literature will be incomplete because it misses those people who stop their meds against advice, and then vow to stay the hell away from psychiatrists for the rest of their born days. Dr Frances paints a scary picture of these poor unfortunates “shamefully neglected in prison dungeons or living on the street”, but is that really true? My own hunch is that most of them successfully make their way in life, seamlessly blending in with the rest of us.”
“Six years ago, against medical advice, I weaned myself off of my medications. My ” time in dungeons and on the streets” occurred while I was on medication. Since going off of my medication, I have been able to go back to school, work full time, and become a productive member of society. I am one of those who will never see a psychiatrist again.”
“I call it a robbery only because anti psychotics, dopamine blockers, neuroleptics what every you want to call them rob the ability to feel any pleasure from things in life. It just blunts it out.”
“I was given anti-psychotics for anxiety when I was 17 years old. I am now 43 and still can’t get off them. Every time I try I become severely psychotic. Then I end up hospitalized and the doctors force me to get back on them. They see the withdrawal as proof that I need them. I was never psychotic in my life until I had been on them for a few years and tried to stop them. I was never mentally ill until I took them. I was just a troubled teenager who needed someone to talk to. I never should have been prescribed anti psychotics.”
“Prior to going on medication, I was miserable, but at least I could hold down a job, socialize and sustain housing. Once I went on drugs, I felt so awful that maintaining a “normal” lifestyle was impossible. The three times I attempted suicide were when I was under the influence of psychotropics. Is that psychiatry’s idea of a “better” outcome?”
27 year old – “I’ve had over 90 different prescriptions go through my young, still-developing brain… the doctors decided my “schizophrenia” was so medication-resistant, that they opted for a tri-weekly injection of the 1st gen major antipsychotic perphenizine. This took only 2 months to give me an Addison’s crisis…I was so ill, my scans and blood tests were off the charts, many 5x the normal levels…my entire endocrine system was shutting down. I had developed Hashimoto’s thyroiditis. My scans showed changes in my pituitary gland…I had to take hormone replacement medication for the rest of my life.”
“Torture, inquisition, Nazi camps, slavery are consistent themes across decades of service user reviews. If by some miracle (political ties, monopoly power), [an industry with such reviews] managed to persist, everybody – consumers, parents, public interest groups – would be clammering [sic] for consumer protection legislation.”
Of course…calling questions and criticisms “anti-psychiatry” may be somewhat effective now, but…my best guess is that the stigma attached to “anti-psychiatry” has lessened, and is lessening, which means that the psychiatrists will eventually have to deal with people and groups who question their practices, tools, beliefs, etc.
Antipsychiatry? Yes. Evil, vindictive and unjustified? I don’t think so.”
Dr Pies sees the writing on the wall and is well aware that people like Robert Whitaker, Phil Hickey and increasingly the public, have his measure so is trying to play both sides why he loses the plot so often? Or is it that Psychiatry has spent so long using PR to bolster its IMAGE instead of critically examining its BASE, that he just doesn’t know what to do?
Report comment
I did not know that a psychiatrist had gone to prison for fraud
Report comment
Pies just offered a response to Whitakers rebuttal. It is on the psychiatric times website underneath their article.
Report comment
I couldn’t find it.
Report comment
Pies and Pierre’s response to Whitaker:
Some Methodological Issues: A Supplementary Statement from Drs. Pierre and Pies:
Our commentary cited six studies of “quality of life” (QOL) and antipsychotics (AP) that involved a placebo control (Hamilton et al, Nasrallah et al, Beasley et al, Leucht et al, Witte et al, and Isitt et al). All these studies found an advantage for AP use vs. placebo, with respect to improving QOL. Like all studies, the ones cited have limitations and methodological shortcomings, and we carefully qualified our conclusions as “provisional”, pending more long-term placebo-controlled studies.
Some critics argue that APs make patients with schizophrenia worse (or even cause “brain damage”), but then simultaneously invoke the speculative notion of “withdrawal” to explain away the results of the studies we have cited, arguing that these studies did not use “true” placebo groups. But if APs actually make patients with schizophrenia worse, then discontinuing the AP ought to make patients better. Conveniently invoking “withdrawal” to explain why, in fact, patients with schizophrenia who are taken off APs do not usually get better is contradictory. (To critics who respond, “Oh, we concede that antipsychotic medications may be helpful in the short term, but they make things worse in the long term,” we would reply that there are no convincing randomized controlled data, either on the basis of relapse frequency or quality of life, that demonstrate that antipsychotics worsen outcome in the long term. As the Sohler et al study concluded; i.e., “Our study did not support the hypothesis that long-term treatment with antipsychotic medication causes harm.” [Am J Orthopsychiatry. December 14, 2015]
Critics also confuse two uses of the term “withdrawal.” One use means, “stopping or discontinuing a drug.” The other refers to withdrawal syndromes, such as the kind experienced when a patient is suddenly taken off a barbiturate or opiate, and experiences shivering, diarrhea, hallucinations, seizures, or even death. There is no such documented “withdrawal” syndrome associated with discontinuation of antipsychotic medications—even sudden discontinuation–except for generally mild and transient cholinergic rebound symptoms, such as hypersalivation, cramps, or diarrhea. These are usually seen within a few days of stopping the AP; are usually mild and self-limited; and rarely need clinical intervention (In rare instances, they are easily managed with anticholinergic medication). These short-term symptoms are quite distinct from a relapse of the illness, which typically occurs 1 or more months after stopping the medication. Thus, the notion that the placebo groups in the studies we cited did worse because they were “in withdrawal” and not “being treated for withdrawal” is completely unsupported by any empirical data.
A “true placebo group” is a group that is given a placebo—period. There is no other definition in the medical literature. It would require an individualized, patient-level analysis in order to demonstrate that any particular patient taking the placebo was “in withdrawal.” We are not aware of any such data. The notion of a “supersensitivity psychosis”, as invoked by some critics, remains only a speculative and theoretical possibility—not, to our knowledge, one confirmed by any biological study (PET, fMRI, etc) of the brains of actual patients.
Finally, while all studies, including the ones we cited, have methodological limitations and potential biases (e.g., pharmaceutical company sponsorship), we have yet to see critics cite a single controlled study, using standard measures of QOL, showing that quality of life improves for patients with schizophrenia when they are no longer taking their antipsychotic medication.
Joseph M. Pierre MD
Ronald W. Pies MD
Report comment
For me longterm Withdrawal Syndrome was a definite reality; and I believe that its this Syndrome that locks people into longterm psychiatric disability (that the initial problems are often psychological but treatment turns them into “imbalaces”).
Tranquilliser Withdrawal Syndrome is a basic medical reality and denying this is not medicine.
I came off drugs very carefully over a number of years but I still suffered from “High Anxiety ” withdrawal syndrome – with symptoms that I had never suffered from before. Thankfully I found effective psychological solutions.
Report comment
Dear me, Ron’s done it again. Set himself up for another demolishing.
“There is no such documented “withdrawal” syndrome associated with discontinuation of antipsychotic medications—even sudden discontinuation–except for generally mild and transient cholinergic rebound symptoms, such as hypersalivation, cramps, or diarrhea.” So much for “many years of experience in treating patients suffering with schizophrenia, our views on antipsychotic medication are shaped not only by our understanding of the scientific literature, but also by our personal care of many hundreds of patients, over several decades.” As I said, they ain’t looking or listening.
Report comment
The option a person is usually given is to stop medication (as I was in my youth). I then ended up in hospital and was diagnosed as a relapser. This happened several times.
I then cut drugs slowly and carefully and followed psychological recommendations and was successful. Withdrawal Syndrome does exist.
Report comment
Yes Robert Whitaker , you have trespassed (lit a candle) revealing a pitch black dark assembly of coercive pseudo – scientific charlatans , DSM thumping self-serving unrepentant unrelenting hoax drug and electrical pushing pharma brown nosers delivering customized individualized tortures to human beings from the cradle to the grave : the psychiatrists , would be titled and respected doctors and prestigious money makers and power wielders the memory of whom will one day live only in infamy as the foremost eugenisist flunkies of Oligarch-Plutocracy applying Therapeutic Technological Weaponized Global Corporatism exclusively of for and by the same truly insane , power wielding ” too big to fail “elite” who evidently feel they must try to crush the human spirit of everyone else , cause they themselves are scared shitless of being seen for what they are and ever so fearful of justice catching up with them . Yes Robert Whitaker, and growing numbers trespass with you together strengthening each other as we go forward as the clarifying light reveals what the darkness is made of . Thank you and others for using your clear sight aiming for the benefit of your fellow human beings .
I meant to say MIA is having an effect . A mental institution facility I visited April of 2016 Sacred Heart in Eugene Oregon had in raised letters on the wall clearly the words BEHAVIORAL CONTROL UNIT. I commented about that here at MIA . 6 months later on Sept. 12 , 2016 visiting the same loved one readmitted to that same unit the sign on the wall had been changed to read BEHAVIORAL HEALTH UNIT . This is not my imagination .I copied the words down carefully with a pen. Also the personal were all a seemingly kind group of female nurses .At the previous visit was a group of wary all male attendants . Not sure what to make of all this . Maybe something to do with the Murphy legislation . Or fear by the facility controllers of follow up inquires and possible negative publicity. Or just a sign of what they plan to do if the Murphy crap passes into law . G-D FORBID.
Report comment
And yet on Sept 8 2016 when I visited the same loved one at Mercy Hospital in Roseburg Or. about an hour expressway Highway 5 drive from Sacred Heart . I saw something “somewhat” different . The ABU GRUB GUANTANAMO ( my name for it) 3 bed crisis unit . The “rooms” are small , stark walls painted a darker shade of eggshell white . No view to the outdoors . A raised 6 ft. by 4ft. waist high platform in the center of the room , a 2 inch mattress in the socket on top the platform . Clean sheets an small pillow on top. There is a switch to turn lights on and off . Camera’s an mics cover entire room . Theres a special room up front attached to small lobby where at least one of two security guards monitor viewing screens at all times .” Meds” are given on time by any means necessary. There is nothing to do . accross the hallway is a room with a toilet and shower . Uniforms are blue strong disposable short sleeve paper pants and top . There is a 20 ft.long 8 ft. wide hallway between the room and the shower room . Sunlight does entirely not enter even indirectly into the space I have described .A paper back book was thrown into the room. Only contact is with staff . It was a really severe sensory depravation torture enviornment my loved had to endure for 7 consecutive days .There were no electrical wires attached to her fingers . Otherwise it reminded me of the photos of ABU GRUB . They did allow me to visit twice . We didn’t know how long this type of torture would be applied .
She was taken there after 10 days at Bay Area Hospital in Coos Bay Oregon followed by a Super Obvious Kangaroo Court finding for a 6 month commitment accused of among other things throwing a half cup of “hot” tea at a psychiatrist not her own as he passed near her at Bay Area Hospital in Coos Bay Or.. I heard the “eminent psychiatrist testify by telephone in the Douglas County Court in Roseburg Or. where they transfered my loved one a 51 year old woman ankles shackled together, belt shackle around waist wrists shackled together at front attached to belt shackle . Just like Hannibal Lector but without the face mask . Public Defender was assigned the case the day before and the Choreographed Kangaroo Court Ritual went as previously planned . Of course I was eventually asked by His Honor the Judge to leave the court room . I complied as two 6 ft.+ armed sheriffs were prepared to drag me out if I didn’t exit. Sadly Roseburg Or. recently went through one of those tragic mass shootings and so they are not so friendly toward anyone with any “diagnosis”. And my sweethearts case manager plus other supervising authorities live and work in Roseburg Or. which is also within Douglas County as is Reedsport ,Or. where we live . Yes I have finally contacted a lawyer that might help if I can come up with the do re mi required . How would of all this gone down with a passed Murphy law . I don’t know and I don’t know where there is to go . But I’m sticking with my love .
Report comment
Oh Yeah , tray of hospital food was also brought into room at meal times at the Roseburg 3 bed Abu Grub crisis unit . It was set on the raised platform bed . Signing off now , Fred
Report comment
THAT’S likely to amend her opinion of psychiatrists, and/or to offer her `therapy’ for the `illness’ that prompted her behavour, isn’t it? If a head-injured football player lashes out as a consequence of his concussion (quite common), is he shackled and confined this way? Does a person with a high fever who curses and throws things at hospital staff cop this? Of course not.
Here in Melbourne Australia in 2015-16 a man’s father instigated an action against the illegal use of electroshock on his son, and the consequences to the son for the next 9 months were, 4 point shackles to a bed on a daily basis, 100 consecutive ECT treatments (3 per week), sanctioned and ordered by the government tribunal and 2 hospital systems, court removal of the father’s custody, total isolation from all but 2 relatives who were never spoken to by staff. This man had never committed any crime but, when goaded, had yelled at staff. He was, at another time, shackled to a bed for 60 days, emerging subdued but unable to walk. (He was in his 30s at the time.) At another time he was confined to a solitary cell with a tray of cat litter for a toilet. He was eventually kidnapped by his friends and family and removed to a state far away where an ETHICAL psychiatrist took his case. Had this not happened I believe he would be dead.
What is happening in our `civilised’ society, that such appalling abuse can happen? What sort of people devise and sanction this? Is mental `health’ run by psychopaths? Was Dostoyevsky right? `When a man has unlimited power over the flesh and blood of his fellow men, when a man is in a position to degrade another human being to the limit of degradation, he is unable to resist the temptation to do wrong. Tyranny is a habit. In the end it becomes a disease. The best man in the world becomes so brutalized as to be indistinguishable from a wild beast’.
That these two eminent self-appointed scientists can see elephant in the room and still say, `I don’t believe it’ confirms my believe that human suffering is the last thing that concerns psychiatry.
Report comment
His name was Garth Daniels and 2 authors wrote in MIA in 2016, documenting the case – Professor John Read and Dr Jock McLaren.
Report comment
As I remember (bare with me now) before Israel became a recognized state by majority vote of other nations the British who were in control were hanging Jewish freedom fighters fighting for independence. Of course to the British they were terrorists . I guess the old cliche” one man’s freedom fighter is another man’s terrorist” would apply to situations like this and others similar disputes. In reply to the capture of members of Urgun and Begin’s organization being hung , the reply was to capture British officers and to hang them simultaneously to the Urgun members being hung .The British were appalled and finally when a large number of British officers were celebrating some event in the King David Hotel it was blown up with many British perishing . Soon after the embarrassed British government and military could no longer justify to their citizens back home in England considering the casualties any reasons for remaining in the middle of a dispute between Holocaust Survivors and other Jew’s on one side and the Arab’s on the other . In any case the British left.
My point : we must somehow along the way make it impossible for psychiatrists to justify to themselves or their family’s that they should continue to go to “work” as psychiatrists and that they leave there G-D forsaken “profession ” in mass numbers and take up some work that does not involve lording themselves over other human beings . Another word’s if they shock us we must shock them , if they drug us into zombie states with poisons likewise for them etc. Along the way we may have to come to this ,otherwise our family’s, our children, our friends, will all of us , when the water in the pot degree by degree gets hot enough, be the frogs that never left the pot as the psychiatrists and pharma and their groupies become ever more emboldened , inventive , and torturous . You got a better idea , I’m all ears .But for G-d sake don’t censor this it’s just one man’s opinion. Tell me what you think we’re supposed to do in this impossible state of affairs , that doesn’t leave us in the frog pot as the temperature rises .
Report comment
Hi Fred
A problem arose in my State with Psychiatrists leaving the public system in large numbers (as a result of the discussions around the introduction of the new Mental Health Act and what it was obvious the aim were, particularly Community Treatment Orders) I got the feeling at the time it was about a claim to high status in our community and that they were not going to be performing the tasks of parole officers who torture and maim with the use of drugs.
The State responded by changing the definition of who could work as a psychiatrist and started seeking people from overseas. These people, like the Pinkertons Detective Agency have come to town and don’t give a damn about health or welfare, they are just here to do the dirty work the State authorities want done, ie increased incarcerations and forced druggings.
And there is not a lot of dissenting voices for fear of being targeted so…….. looks like were up for a few cooked frogs where I live.
Report comment
And I had the most revealing conversation with a member of Parliament about the new MHA after showing him the quality work being done to bring ‘treatment’ to those in our community who require it.
Question 1: Given that the grounds required for detention and forced drugging are missing a couple of days sleep and not eating properly, when are we going to start locking up Muslims during Ramadan and ensuring they receive the ‘treatment’ they would not accept willingly. No answer, just a brushing off.
Question 2: Given that the UN has stated that Australian MH laws are a violation of human and civil rights, and that this statement by the UN has been totally ignored, what is being done to correct this situation? No answer.
Question 3: the party line about the new MHA is that it has “added protections” might it not be important that the community is aware that these protections are to ensure that doctors have carte blanche to do whatever they deem necessary, with zero legal protections for anyone with the status of “patient”? No answer.
I can see how mercenaries will work. And the people being brutalised will be forced to pay for it.
Report comment
This does not bode well given our Minister for Mental Health is authorising the drugging without knowledge and planting of knives on citizens to obtain police referrals to Mental Health, who then remove any human and civil rights by making the citizen a “patient” and begin treatment.
Once designated “patient” the sort of ‘treatment’ given to Garth Daniels is ….. therapuetic.
Report comment
Think they would sue me for saying such things outside parliamentary privilege, but the Minister would be aware that police are being used to deny me access to my property, to retrieve the documentary evidence, and to threaten and intimidate anyone who knows the facts relating to these matters.
Anyone know a good journalist? lol
Report comment
And with such solid reasoning as the argument from authority from the Minister and the Chief Psychiatrist as ‘we had reasonable grounds to believe the citizen required incarceration and forced drugging because we went to the trouble of making it up’? What chance has anyone got waaahahahaha
Report comment
Wow, there are a lot of comments here, all preaching to the choir, at least as far as I got through them.
Nothing is going to change. It doesn’t matter whose right or who isn’t. Power is power. It exists independent of what is right. That’s how we got here in the first place.
Report comment
You’re absolutely right & Lord Acton’s old saw remains – “All power corrupts and absolute power corrupts absolutely”, hence tyranny, “which is usually thought of as cruel and oppressive, and it often is, but the original definition of the term was rule by persons who lack legitimacy, whether they be malign or benevolent. Historically, benign tyrannies have tended to be insecure, and to try to maintain their power by becoming increasingly oppressive.”
From Sorokin and Lunden:
1.” When the morality and mentality of rulers and the ruled are measured by the same moral and mental yardstick then the rulers’ morality and minds appear to be marked by a much stronger dualism –
2.The ruling ,groups contain a larger proportion of the extreme mental types of the gifted and the mentally sick than the rank and file of the ruled population
3. The moral behaviour of ruling groups tends to be more criminal and sub-moral than that of the ruled strata of the same society.
4. The greater, more absolute, and coercive the power of rulers, political leaders, and big executives of business, labour and other organizations, and the less freely this power is approved by the ruled population, the more corrupt and criminal such ruling groups and executives tend to be.
5. With a progressive limitation of their power, criminality of rulers and executives tends to decrease qualitatively (by becoming less grave and murderous) and quantitatively (by decreasing the rate of criminal actions)
There is no doubt that the occupational functions of rulers contain several morally ennobling activities such as: the protection of life, security, freedom and other rights of the citizens;…As a rule, this moral excellence is confined within a limited portion of rulers’ mentality and behaviour, beyond which they may remain immoral, even criminal… activities of rulers deaden their moral sensitivity, and harden their souls and hearts towards the lives and values of human beings…. eliminating possible competitors; suppressing disorders, riots and revolts; punishing the violators of law; sacrificing many an “expendable” human life… activities are sometimes performed for the protection and expansion of selfish interests…organized spying, skilful lies, hypocritical assurances, false promises, threats and bribes, semi-rational persuasions, limited coercion, cloak and dagger actions, cynical machinations, and other morally doubtful procedures.
power generates in them (and in others, too), a belief that they are the chosen and anointed who are far above the ruled population and its common-herd moral and legal precepts of right and wrong, good and evil…In brief, they see themselves as essentially free from the limitations of unpleasant legal obligations and moral imperatives. Such a freedom amounts to a moral and legal nihilism.”
BUT – from Dr Mohammad Omar Farooq:
Whether it is the world’s court of justice or not, history does record rather consistently that tyranny and abusive powers don’t last. It is just as true for individual tyrants as it is for tyrannical regimes.
Empirically speaking, all the empires of the past that were based on tyranny or the abuse of power, subverting the common bond of humanity, have fallen from their heights – sometimes to their nadir or became extinct.”
BUT , in avoiding tyranny people must never acquiesce…Failure to take corrective action early will only mean that more severe measures will have to be taken later, perhaps with the loss of life and the disruption of the society in ways from which recovery may take centuries.”
Have we let psychiatry go too far for too long? Is there so much money and power that it will take `centuries’ to bring it down? The attack on our children is getting more and more evident – is this incidental or…?
I’m glad I’m old because the thought of the world in 50 years terrifies me. The Brave New World is already happening.
Report comment
Aldous Huxley’s `Brave New World’ and Geiorge Orwell’s `1984′ should by compulsory prescribed texts from primary school up, and should be re-read every single year.
Report comment
But as it happens forcing will ‘kill’ the meaning. Ideas also can be used like external drugs or interventions and coercions. The devil quotes Scripture.
A culture of awakened responsibility cannot be replaced by any set of ideas, methods or identities and persist AS awakened responsibility – aka a true sense of shared integral worth.
Education is a guidance in the bringing forth of the willingness and focus of the individual so as to balance learning and navigating the world with finding fulfilment in what they are moved to discover and become.
Seeing it as ‘engineering good or bad’ outcomes is an example of how power struggle corrupts everything to serve a war-minded sense of self.
Report comment
Somewhere a child is suffering “EPS”, dystonia,the inner muscles s of their mouth lock up, and their chest. They can struggle to swallow, struggle to breath. The nurses will call it “EPS”, a side effect of the “medication” and float their way one through their job disregarding you because you had the benadryl and cogentin. People need to stop talking and start acting. Power is power. Words have no power. No child should suffer what I went through.
Report comment
Pies has added yet another clarification regarding withdrawals on the psych times website.
Report comment
Such regulatory capture as corporates seek to enforce – and often do – is not really about science or any other desire to ascertain truth. It is all about power – All about identity in a sense of power that is rooted in powerlessness – or it would rest in its power and be open to communication – rather than protect its exclusive rights against threats that reveal its lack of true foundation.
Szatz was largely correct – but not understood. Not that dis-ease does not manifest as very real disturbance or breakdown of function – but that the primary cause and farming of this is not in chemical or organic effects – though increasingly in this era they are serving as cofactors or pathways of a negative synergy. A list of suspects in such would include herbicides, pesticides, hormone disruptors, prescription drugs that block vital functions such as cholesterol, or destroy gut bacteria, malnutrition due to de-natured food. and I’m sure the list goes on. But of course coming under prescription for anti depressents and similar is itself a major reinforcement to the root cause.
The nature of the cause is indicated by the stories of those who come through whatever hell they were caught up in to live from a more awakened responsibility. there is not a cause that one can make war on – though there are many negative reinforcers that can be addressed as putting obstacles in the way rather than helping. But the cause of disease is operating against or attempting to override our true nature – and politicising health under a pharma captured ‘medical’ regulatory state is merely extending the cause of sickness in a form that purports to be treating it. The lie is the usurping of the true but the father of the lie is wanting it to be true. However if people are mis-educated to only believe in pharma as genuine and effective – then of course they will ‘want’ it or at least not challenge it – unless they have a strong intuitive sense of their Life – or have gained an education despite schooling.
The phrase ‘post truth politics’ seems apt fro the REFUSAL to engage in any but a FORM of dialog and for the purpose of obfuscation, sowing doubt, underming or defaming the ‘rival’ narrative. In fact all the things that tax dollars are spent on training disinfo operatives to go out and ‘protect our national security’ against threat.
One cannot engage with a troll but to be trolled – though one may be able to use any public arena of discourse to bear witness to truth – including calling out such tricks as lacking any substance and unworthy of responding as if they had.
Report comment